AP BIO UNIT 1: chemistry of life
0.0(0)
0.0(0)
New
Card Sorting
1/28
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
1
New cards
topic 3.
**WATER**
**WATER**
**OVERVIEW:**
* **water and the importance of hydrogen bonds.**
* **pH**
* **water and the importance of hydrogen bonds.**
* **pH**
2
New cards
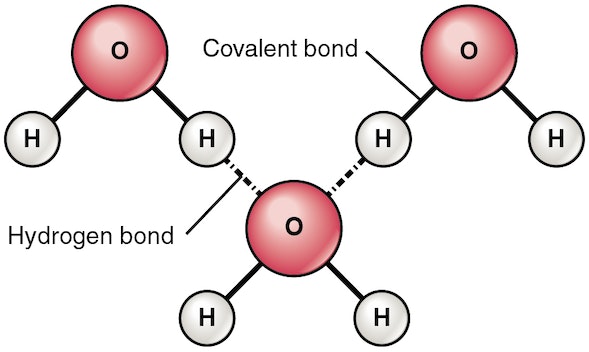
hydrogen and covalent bonds
\
3
New cards
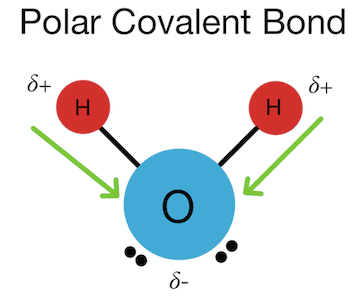
polar covalent bond
a partial negative charge around the oxygen atom and a partial positive charge around the hydrogen atom.
4
New cards
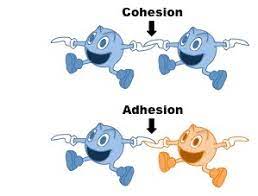
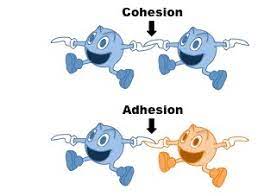
adhesion
different molecules attract each other.
5
New cards
cohesion
like molecules attract each other.
6
New cards
high specific heat:
as a result of water’s ability to form hydrogen bonds, more energy is required to separate water molecules during phase changes, giving water a high specific heat.
7
New cards
moderating climate:
since water has a high heat capacity, it can absorb and release large amounts of energy. this stabilizes climates in locations near large bodies of water.
8
New cards
expanding upon freezing:
since water has the ability to form hydrogen bonds, there is more space between water molecules in the solid state than in the liquid state. as a result, ice had a lower density than that of liquid water, and thus ice floats on liquid water.
9
New cards
acting as a great solvent for other polar molecules and for ions:
water has a partially positive end and a partially negative end. thus, water can readily dissolve ionic compounds and other polar molecules.
10
New cards
pH
measures the concentration H+ ions in a solution.
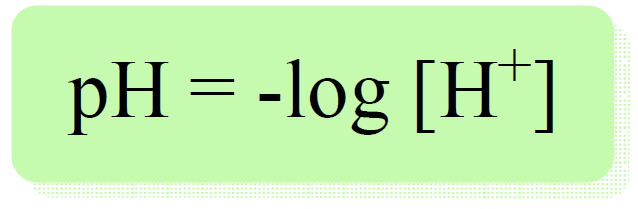
11
New cards
buffers
crucial in maintaining relatively constant pH levels in living cells. buffers can form acids or bases in response to changing pH levels in a cell.
12
New cards
topic 4.
**MACROMOLECULES**
**MACROMOLECULES**
**OVERVIEW:**
* **biological macromolecules**
* **protein structure**
* **nucleic acids**
* **biological macromolecules**
* **protein structure**
* **nucleic acids**
13
New cards
macromolecules necessary for life are primarily made up of six elements…
nitrogen, carbon, hydrogen, oxygen, phosphorus, and sulfur.
14
New cards
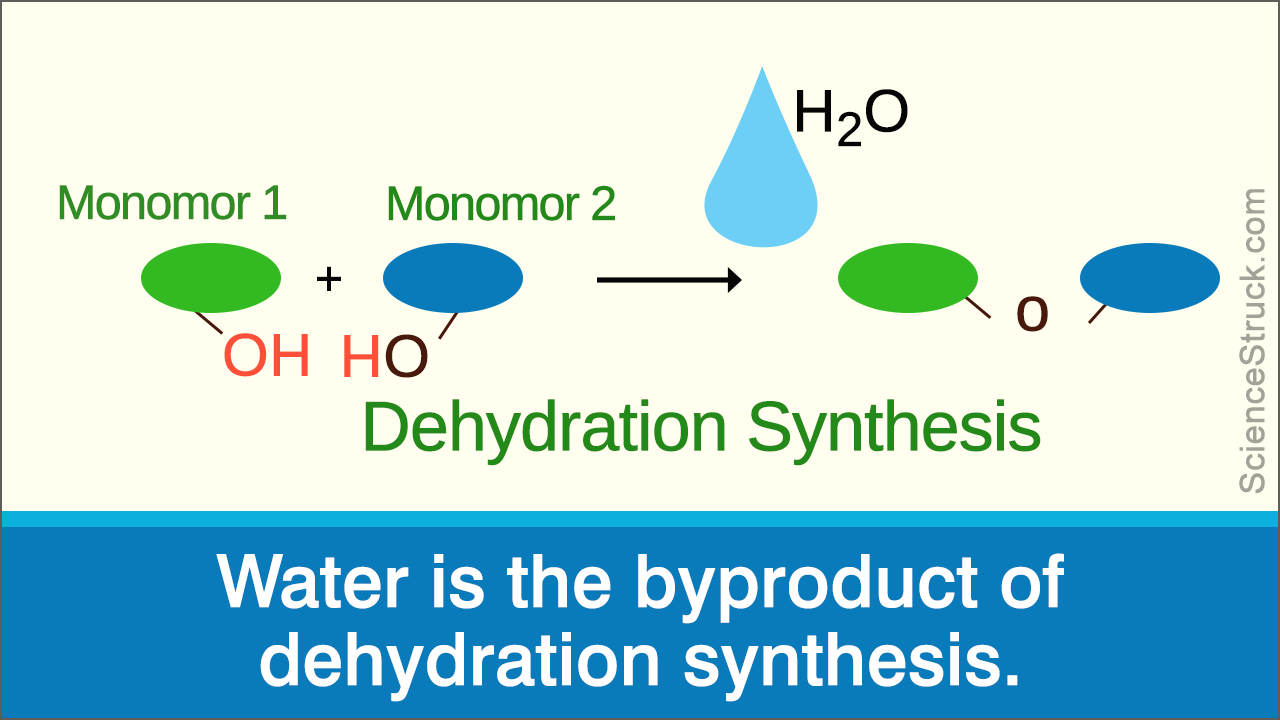
dehydration synthesis
biological macromolecules are formed from building blocks (i.e., monomers) that are linked by dehydration synthesis.
15
New cards
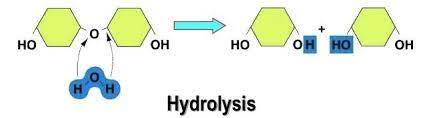
hydrolysis
these biological macromolecules are broken down by hydrolysis reactions.
16
New cards
carbohydrates
* polymers of sugar monomers
* used to store energy (such as starch or glycogen)
* can also have structural functions (such as cellulose)
* linkage: glycosidic bond or glycosidic linkage
* used to store energy (such as starch or glycogen)
* can also have structural functions (such as cellulose)
* linkage: glycosidic bond or glycosidic linkage
17
New cards
lipids
* building blocks: fatty acids
* nonpolar macromolecules that function in energy storage, cell membranes, and insulation.
* linkage: ester linkages
* nonpolar macromolecules that function in energy storage, cell membranes, and insulation.
* linkage: ester linkages
18
New cards
unsaturated
fatty acids with at least one C=C double bond are called unsaturated, are liquid at room temperature, and usually originate in plants.
19
New cards
phospholipids
they are built from a glycerol molecule, two fatty acids, and a phosphate group. because fatty acids are nonpolar and the phosphate is polar, phosphlipids are amphipathic, meaning that they have both hydrophobic and hydrophilic regions.
20
New cards
nucleic acids
* polymers of nucleotides
* carriers of genetic information
* linkage: phosphodiester linkage
* carriers of genetic information
* linkage: phosphodiester linkage
21
New cards
proteins
* polymers of amino acids
* have an amino group, a carboxylic acid group, a hydrogen atom, and a side chain (r-group) attached to a central carbon.
* functions in enzyme catalysis, maintaining cell structures, cell signaling, cell recognition, and more.
* linkage: covalent peptide bond
* have an amino group, a carboxylic acid group, a hydrogen atom, and a side chain (r-group) attached to a central carbon.
* functions in enzyme catalysis, maintaining cell structures, cell signaling, cell recognition, and more.
* linkage: covalent peptide bond
22
New cards
r-group
unique for each amino acid; it determines the amino acid’s identity and whether the amino acid will be nonpolar, polar, acidic, or basic.
23
New cards
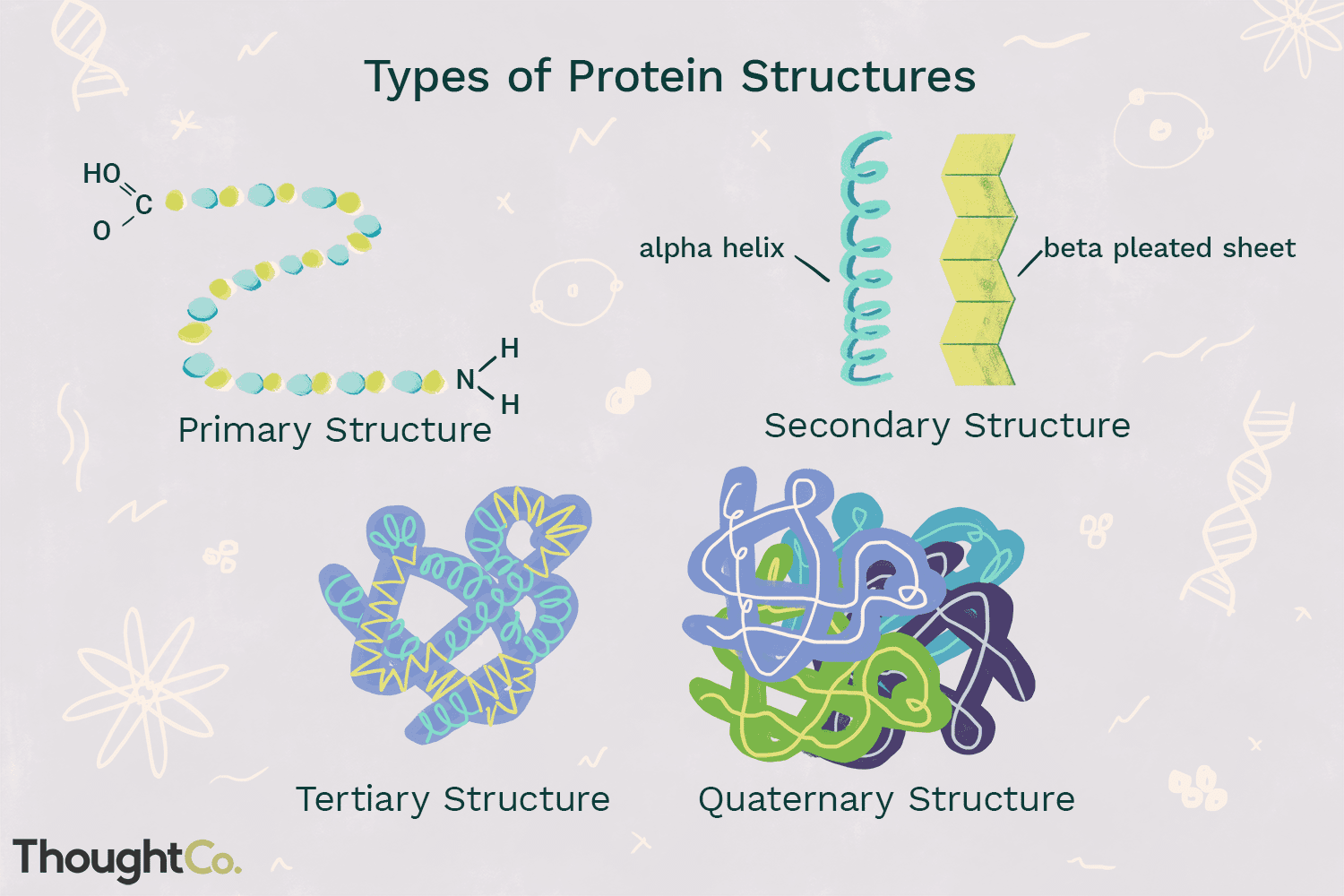
protein structure
there are four levels of protein structure: primary, secondary, tertiary, and quaternary.
24
New cards
primary structure
amino acids are joined by peptide bonds. The resulting polypeptide chains have directionality, with an amino (NH2) terminus and a carboxyl (COOH) terminus. the order of the amino acids in the polypeptide chain determines the primary structure of the protein.
25
New cards
secondary structure
one the primary structure is formed, hydrogen bonds may form between adjacent amino acids in the polypeptide chain. this drives the formation of the secondary structure of the protein. these secondary structures include alpha helixes and beta-pleated sheets.
26
New cards
tertiary structure
the three-dimensional folded shape of a protein, often determined by the hydrophobic/hydrophilic interactions between r-groups in the polypeptide.
27
New cards
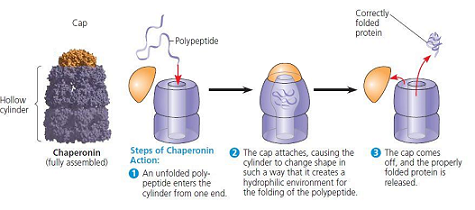
chaperonins
special proteins called chaperonins often help fold a polypeptide into its three-dimensional structure.
28
New cards
quaternary structure
some proteins consist of multiple polypeptide chains (subunits), which are joined together to form the complete protein and function as a unit.
29
New cards
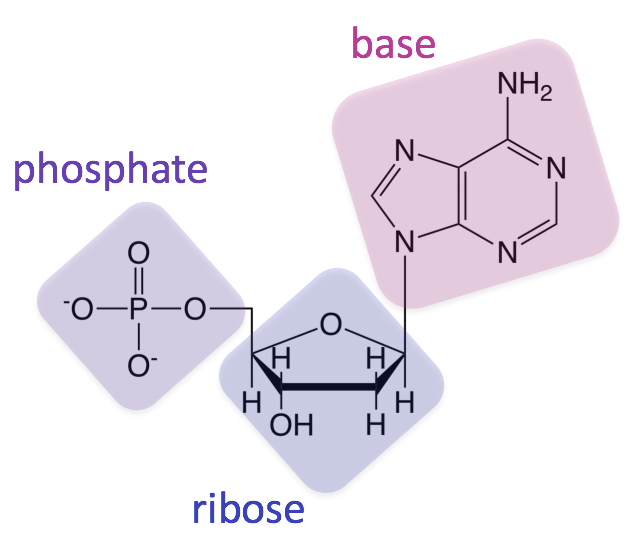
nucleic acids
(DNA and RNA) are polymers of nucleotides. the genetic information is stored and communicated through the order of these nucleotides. nucleotides consist of a five-carbon sugar (deoxyribose or ribose), a nitrogenous base (adenine, thymine, cytosine, guanine, or uracil), and a phosphate group. nucleotides have directionality in that the phosphate group is always attached to the 5’ carbon in the sugar, and the 3’ carbon always has a hydroxyl group to which new nucleotides may be added.