Arenes_Concepts
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Why do benzenes prefer to undergo substitution rather than addition reactions?
Delocalisation of the 6π electrons in the continuously overlapping P orbitals confers resonance stability on benzene
If benzene undergoes addition reactions, overall aromatic character is destroyed
If benzene undergoes substitution reactions, aromatic character is retained
General steps of electrophilic substitution mechanism
Generation of electrophile, E+
Electrophilic attack by E+ on the benzene ring to form a carbocation (slow step)
Loss of proton from the carbocation to form the product (fast step)
Difference in conditions of nitration of benzene and methylbenzene
Benzene: 50˚C
Methylbenzene: 30˚C
A Bronsted-Lowry acid is a proton ___
Donor
A Bronsted-Lowry base is a proton ___
Acceptor
Eqn of generation of electrophile, NO2+ in nitration of benzene
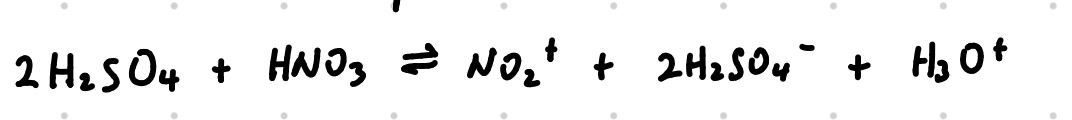
A Lewis acid is an electron pair ___
Acceptor
A Lewis base is an electron pair ___
Donor
Activating groups
Electron-donating
Increases electron density in benzene ring, makes ring more susceptible to electrophilic attack
Helps to disperse the positive charge in the intermediate carbocation and stabilise carbocation
Makes substituted benzene more reactive than benzene
Deactivating groups
Electron withdrawing
Decreases electron density in benzene ring and makes the ring less susceptible to electrophilic attack
Intensifies positive charge in intermediate carbocation, destabilise carbocation
Makes substituted benzene less reactive than benzene
Criteria for oxidation of side chains on benzene
Benzylic C atom must have at least one H or O atom bonded to it