M4 - Antibody Identification Methods Antiglobulin Test Titration, Elution & Adsorption
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Define antibody titre, affinity, and avidity.
Antibody titre: The concentration of antibody present in a person’s plasma.
Affinity: The strength of a single antibody-antigen interaction.
Avidity: The overall functional strength of binding when multiple antibody sites interact with antigens.
What is thermal amplitude and why is it clinically relevant in blood banking?
Thermal amplitude: The temperature range over which an antibody can bind to its corresponding antigen.
Clinical relevance: Determines whether an antibody reacts at body temperature (potentially causing hemolysis) or only at cold temperatures (usually clinically insignificant).
Describe the role of the complement system in antibody-mediated hemolysis.
Complement proteins circulate in inactive forms and become sequentially activated in a cascade.
Activation can be triggered when antibodies bind to antigens on red cells.
IgG subclasses 1, 2, and 3 can activate complement, leading to red cell lysis.
IgG4 usually does not activate complement.
Which IgG subclasses are effective at activating complement, and which are not?
Effective: IgG1, IgG2, IgG3
Ineffective: IgG4
Explain how complement contributes to the immune defense against foreign red cells.
Antibodies bind foreign red cell antigens.
This binding triggers the complement cascade, leading to formation of the membrane attack complex (MAC).
The MAC creates pores in the red cell membrane, causing lysis and removal of the foreign cell from circulation.
Why was the AHG test developed?
Prior to the AHG test, only IgM antibodies could be detected because they cause direct agglutination.
IgG antibodies are nonagglutinating (“incomplete”) and require AHG to detect sensitized RBCs.
Coombs and coworkers (1945–1946) developed the procedure to detect IgG antibodies and complement on RBCs.
What is the purpose of the AHG test in blood banking?
Detect RBCs sensitized with:
IgG alloantibodies
IgG autoantibodies
Complement components (e.g., C3d)
What are the sources of AHG and how are they produced?
Sources: Rabbit, goat, sheep (especially for large volumes).
Production: Animals are immunized with human globulins.
One colony immunized with human IgG → produces anti-IgG AHG.
Another colony immunized with human complement (C3) → produces anti-complement AHG.
Distinguish between polyspecific and monospecific AHG reagents.
DPolyspecific AHG: Contains antibodies against human IgG and complement (C3d).
Monospecific AHG: Contains antibodies against either IgG or complement, but not both.
Compare polyclonal and monoclonal AHG reagents.
Polyclonal AHG:
Recognizes multiple epitopes on the target antigen.
Broad reactivity.
Monoclonal AHG:
Recognizes one specific epitope.
Advantages: Pure, consistent specificity.
Disadvantages: Cannot detect other epitopes, may miss some antibody types.
Why is AHG reagent not required to detect IgM antibodies?
IgM antibodies are large and pentameric, causing direct agglutination of RBCs at room temperature.
AHG is only needed for IgG antibodies, which are small and do not directly agglutinate RBCs.
What are the two applications of the AHG test?
In vivo sensitization: Direct Antiglobulin Test (DAT)
One-stage procedure detecting antibodies or complement already bound to patient RBCs.
In vitro sensitization: Indirect Antiglobulin Test (IAT)
Two-stage procedure detecting antibodies or complement coated on RBCs during testing.
Which activities must AHG contain to be effective?
Anti-IgG activity (primarily IgG1 and IgG3 subclasses)
Anti-complement activity (detects complement components such as C3d)
Clinically significant antibodies detected include anti-ABO, anti-Lewis, anti-Kidd, anti-P, etc.
Compare polyspecific and monospecific AHG in the IAT.
Polyspecific: Detects both IgG and complement (C3d).
Monospecific: Detects either IgG or complement, but not both.
Choice depends on clinical context; polyspecific is commonly used, monospecific can help clarify results.
How do potentiators like LISS and Albumin influence the IAT?
LISS (Low Ionic Strength Solution): Lowers zeta potential, disperses negative charges, enhancing agglutination for some antibodies.
Albumin: Enhances RBC sensitization differently, can detect antibodies that LISS may miss.
Example: Anti-K (clinically significant HTR) may not be detected with LISS but detected with albumin.
LISS may also show false positives due to cold, clinically insignificant antibodies.
Define the Direct Antiglobulin Test (DAT) and its primary clinical applications.
Define the Direct Antiglobulin Test (DAT) and its primary clinical applications.
Define the Indirect Antiglobulin Test (IAT) and its primary clinical applications.
IAT detects in vitro sensitization of RBCs with antibodies from patient serum.
Applications:
Pre-transfusion compatibility testing
Prevention of HDFN
RBC phenotyping
Titration of incomplete antibodies
Explain why IgM antibodies do not require AHG reagent for detection.
IgM antibodies are naturally agglutinating, so they can directly cause RBC agglutination. AHG reagent is required only for IgG antibodies, which are too small to cause visible agglutination without bridging.
What is the difference between polyspecific and monospecific AHG reagents?
Polyspecific AHG: Contains antibodies to both IgG and complement (C3d).
Monospecific AHG: Contains antibody to either IgG or complement only.
List the key factors affecting the sensitivity of the AHG test.
Number of IgG or complement molecules on RBCs
Serum-to-cell ratio
Reaction medium (Albumin, LISS, PEG)
Temperature
Incubation time
Proper washing of RBCs
Addition of AHG reagent
Centrifugation for reading
List the most common sources of error in the AHG test.
Inadequate washing of RBCs
Nonreactive AHG reagent
Failure to add AHG reagent
Note: All negative antiglobulin test reactions must be checked by adding IgG-sensitized cells to confirm reagent activity.
Explain the principle of the low ionic polybrene (LIP) technique.
Polybrene is a rouleaux-forming reagent that enhances the crosslinking of sensitized RBCs, allowing detection of IgG antibodies more efficiently.
Describe the enzyme-linked antiglobulin test (ELAT) and its use.
Enzyme-linked AHG is used to detect IgG-sensitized RBCs.
The enzyme amplifies the signal by producing a visible reaction when IgG is bound to RBCs.
What is the principle of the gel method in AHG testing? List its advantages and disadvantages.
Principle: RBCs are added to gel microcolumns containing AHG; sensitized cells are trapped in the gel, forming a visible band.
Advantages:
More sensitive DAT
No washing steps required
Small test volume
Can be automated
Stable end points
Disadvantages:
Warm autoantibodies may be enhanced
Increased cost
Detection of clinically insignificant antibodies
Requires additional equipment
Compare the saline tube method vs. LISS tube method for AHG testing.
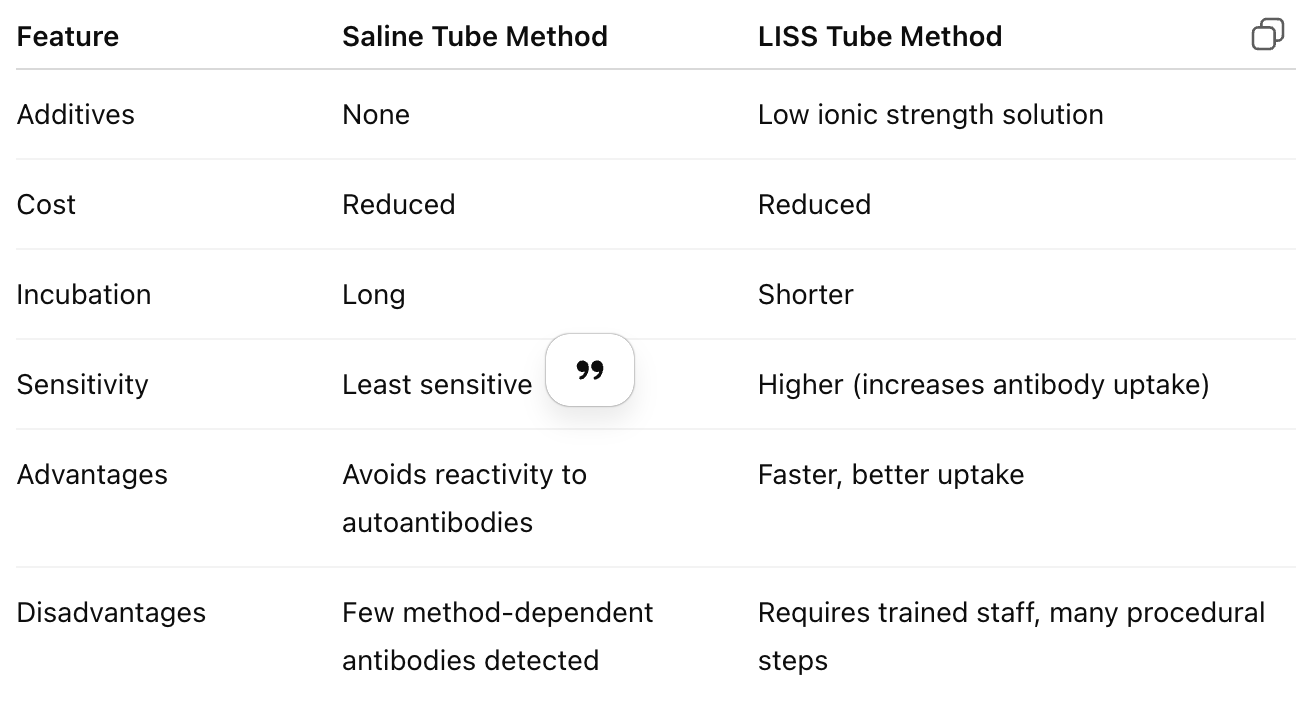
Why is it important to check all negative AHG reactions with IgG-sensitized cells?
To ensure that the AHG reagent is active and that a true negative reaction is obtained, not a false negative due to reagent failure or procedural error.