Cell Biology 1
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
103 Terms
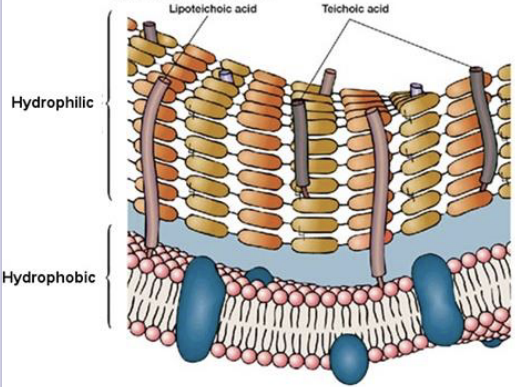
This membrane is from what type of bacteria?
A gram + bacteria.
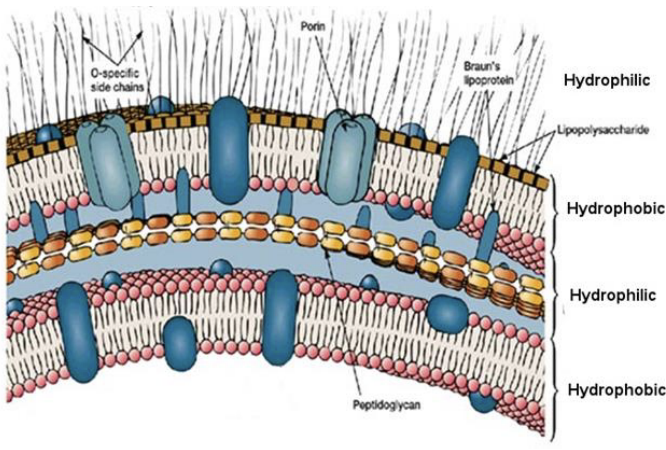
This membrane is from what type of bacteria?
A gram - bacteria.
On average, what is the composition of a membrane?
50% lipid to 50% protein.
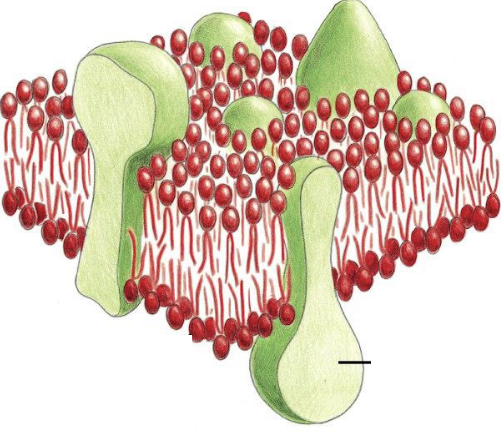
What are the green blobs in this image?
Protein molecules.
What effect does cholesterol give on the packing ability of lipids?
It increases.
What effect does cholesterol have on the membranes relationship to water soluble molecules?
It reduces the permeability to water soluble molecules.
What effect does cholesterol have on fluidity of the membrane?
It does not make the membrane less fluid.
What is the fundamental purpose of membranes in living systems?
To compartmentalize reactions and control conditions within the cell.
Which best describes the term ‘amphipathic’?
A molecule with both hydrophilic and hydrophobic regions.
What is the approximate thickness of a phospholipid bilayer?
5 nanometers.
In cell membranes, approximately what percentage of the material can be protein?
50%
In fungi, which molecule serves a role analogous to cholesterol in animal membranes?
Ergosterol
What is the primary structural difference between gram-positive and gram-negative bacteria?
Gram-positive have one membrane; gram-negative have two.
Why is “flipping” phospholipids across the bilayer energetically difficult?
It moves polar groups through a hydrophobic region.
Which proteins assist in the flipping of phospholipids across the bilayer?
Flipases and scramblases.
How does temperature affect membrane fluidity?
Increasing temperature increases molecular motion and fluidity.
What is the major effect of saturation level in fatty acid tails on membrane properties?
More saturated tails make the membrane more rigid.
What are lipid rafts?
Temporarily organized membrane microdomains enriched in cholesterol and sphingolipids.
Which protein is especially associated with lipid raft formation?
Caveolin
What is the major signaling role of inositol phospholipids in membranes?
They serve as precursors for secondary messengers like IP₃ and DAG.
What experimental model did the lecturer mention for studying controlled membrane systems?
Micelles
What is the key difference between eukaryotic and prokaryotic membrane systems?
Eukaryotes have internal, membrane-bound organelles.
Why are membranes considered essential for life’s origin?
They allowed self-contained chemical reactions within micelles.
What is meant by membrane “fluidity”?
The ease with which phospholipids and proteins move laterally.
What is the main reason water permeability is reduced by cholesterol?
Cholesterol increases lipid tail packing density.
What best summarizes the professor’s view of what defines life?
Compartmentalized reactions enclosed by membranes.
According to the endosymbiotic theory, mitochondria originated when:
An archaeal cell engulfed a proteobacterium capable of ATP production.
What is the main evidence that mitochondria were once free-living bacteria?
They have their own circular DNA and ribosomes similar to bacteria.
Chloroplasts are believed to have originated from:
A eukaryotic cell engulfing a cyanobacterium (blue-green algae).
The endosymbiotic events that gave rise to mitochondria and chloroplasts are thought to have occurred:
Only a few times in Earth’s history.
Which feature distinguishes eukaryotic cells from prokaryotic cells?
Presence of membrane-bound organelles.
What does the Golgi apparatus do with lipids and proteins destined for the plasma membrane?
Glycosylates them and directs them so their non-cytosolic face becomes extracellular.
The appearance of phosphatidylserine on the outer leaflet of a cell membrane signals:
Cell stress or apoptosis.
Lipid rafts are specialized membrane microdomains that:
Are enriched in cholesterol and sphingomyelin for signaling.
Cholera toxin causes severe diarrhea by:
Increasing cAMP, activating CFTR, and pumping Cl⁻ ions out of cells.
Mucinex produces its effect in the throat by:
Pharmacologically mimicking cholera toxin to increase water secretion.
A transmembrane alpha helix is most likely composed of:
Hydrophobic amino acids.
The classic example of a seven-transmembrane protein is:
Rhodopsin (light-sensitive GPCR).
What structural change in rhodopsin allows light detection?
Cis-retinal converts to trans-retinal upon photon absorption.
Beta barrel membrane proteins are commonly found in:
Bacterial outer membranes, mitochondria, and chloroplasts.
SDS (sodium dodecyl sulfate) is best described as:
A strong ionic detergent that denatures proteins and solubilizes membranes.
Triton X-100 is used in biochemistry to:
Gently solubilize membranes while preserving organelle integrity.
Why are membrane proteins difficult to crystallize for structural studies?
They are amphipathic and require detergents to stay soluble.
Pore-forming bacterial toxins kill cells by:
Inserting beta-barrel structures that disrupt membrane integrity.
Which immune cell mechanism mirrors the bacterial pore-forming process?
Cytotoxic T cells release perforin to form pores and deliver granzymes.
Loss of membrane potential across the cell membrane signifies:
Cell death, due to inability to maintain ion gradients and ATP synthesis.
What is the primary function of membrane compartmentalization in cells?
To isolate biochemical reactions and allow controlled environments.
Which of the following statements about lipid bilayers is true?
Lateral movement of lipids within a leaflet is common and energetically favorable.
What type of proteins assist in moving phospholipids from one leaflet of the bilayer to the other?
Flippases and scramblases.
What is the effect of cholesterol on membrane structure?
Increases packing of lipid tails and decreases permeability.
In fungi, which molecule serves a similar structural role to cholesterol in animal membranes?
Ergosterol
What structural feature makes phospholipids amphipathic?
A hydrophilic head and hydrophobic tails.
What happens when enough phospholipids self-assemble in water?
They form micelles or bilayers due to hydrophobic interactions.
The fluidity of a membrane increases when:
Fatty acid chains are shorter and more unsaturated.
Which statement about lipid rafts is most accurate?
They are transient, cholesterol-rich microdomains important for signaling.
Which protein is known to be important in lipid raft formation?
Caveolin (Caviolin)
What role does cholesterol play in lipid raft formation?
It promotes tight packing of lipids, allowing microdomain coalescence.
How do prokaryotic and eukaryotic cells differ in terms of membrane-bound structures?
Eukaryotes have membrane-bound organelles; prokaryotes do not.
What is meant by “bilayer asymmetry”?
The inner and outer leaflets have different lipid and protein distributions.
Which factor directly increases membrane rigidity?
Long, saturated fatty acid chains.
In the GPCR-phospholipase C signaling pathway, cleavage of PIP₂ produces:
DAG and IP₃
What does diacylglycerol (DAG) do after PIP₂ cleavage?
It serves as a docking site for protein activation.
What term describes temporary, organized lipid-protein patches that facilitate signaling?
Lipid rafts
Why is it energetically unfavorable for a phospholipid to “flip” from one leaflet to another?
The polar head must cross the hydrophobic core of the membrane.
What is the approximate size of a typical mammalian epithelial cell compared to a bacterium?
About 15–20 microns, roughly 1,000 times the volume of a 1-micron bacterium.
The nucleus is surrounded by what structure?
A double membrane continuous with the endoplasmic reticulum.
Why is the ER described as the “birthplace” of many cellular components?
Because many proteins are synthesized and folded there before moving elsewhere.
What evolutionary insight does the “membrane invagination” model of the eukaryotic cell provide?
That the lumens of organelles are topologically equivalent to the extracellular space.
What is the primary mechanism for transferring large molecules between organelles?
Vesicular transport involving budding and fusion.
Which organelle’s proteins are typically synthesized inside the organelle rather than in the cytosol?
Mitochondria
What directs a protein to its proper cellular destination?
Signal sequences or signal patches on the protein.
What distinguishes a signal patch from a signal sequence?
Signal patches form from discontinuous regions that come together after folding.
What type of signal keeps a protein retained in the endoplasmic reticulum?
KDEL (Lys-Asp-Glu-Leu) C-terminal retention signal
How do most proteins enter the nucleus from the cytosol?
Through nuclear pores using nuclear localization signals (NLS) and import receptors.
What small GTPase creates the energy gradient driving nuclear import and export?
Ran (RAN-GTP / RAN-GDP cycle)
Where is Ran-GEF (guanine exchange factor) localized, and what does it do?
In the nucleus; converts Ran-GDP to Ran-GTP.
How does the cell ensure unidirectional movement of a protein into the mitochondrial matrix?
Chaperone binding and electrostatic attraction prevent backsliding (ratchet mechanism).
What translocators are required to import proteins fully into the mitochondrial matrix?
TOM (outer membrane) and TIM (inner membrane) complexes
What type of sequence halts translocation and embeds a protein in the mitochondrial inner membrane?
Stop-transfer sequence
What is the function of Hsp70 and similar chaperone proteins in mitochondrial import?
Prevent premature folding and assist translocation through the pore.
How do peroxisomes typically receive their matrix proteins?
Through post-translational import of fully folded proteins.
What is the main toxic byproduct generated by peroxisomal oxidation reactions?
Hydrogen peroxide (H₂O₂)
How do new peroxisomes form?
By budding from the ER and division of pre-existing peroxisomes.
Which of the following best describes co-translational transport into the ER?
Protein synthesis occurs simultaneously with translocation of the nascent chain into the ER lumen.
What key feature allows a ribosome to attach to the ER membrane during co-translational transport?
An N-terminal signal peptide recognized by the signal recognition particle (SRP).
In the in-vitro translation + microsome experiment, what does protease resistance of a newly synthesized protein indicate?
The protein successfully entered the lumen of the microsomes.
In the same in-vitro system, adding detergent before protease treatment serves as a control for:
The activity of the protease in degrading accessible proteins.
The signal recognition particle (SRP) temporarily halts translation in order to:
Allow time for the ribosome–nascent chain complex to dock with the ER membrane.
The Sec61 translocon channel remains closed when inactive. Why is this important?
To prevent uncontrolled diffusion of calcium and other ER contents into the cytosol.
The energy that drives the movement of a growing polypeptide into the ER lumen primarily comes from:
The elongation of the peptide by the ribosome.
Which of the following best defines a stop-transfer sequence?
A hydrophobic region that halts translocation and anchors the protein in the membrane.
A single-pass transmembrane protein synthesized in the rough ER will ultimately display which of the following orientations at the plasma membrane?
The luminal domain becomes extracellular.
Which amino acid sequence motif is typically recognized for N-linked glycosylation in the ER?
Asn–X–Ser/Thr
The calnexin cycle in the ER lumen functions primarily to:
Bind monoglucosylated glycoproteins and allow them time to fold properly.
What happens to severely misfolded proteins in the ER that cannot achieve proper conformation?
They are retro-translocated into the cytosol and degraded by the proteasome.
Which process removes sugars from misfolded proteins before proteasomal degradation?
N-glycanase
The unfolded protein response (UPR) serves to:
Increase ER size and folding capacity, while reducing overall translation.
During ER stress, the PERK pathway helps the cell by:
Phosphorylating eIF2α to globally reduce protein synthesis.
The IRE1 branch of the UPR has an unusual feature because it:
Acts as a ribonuclease that splices mRNA in the cytosol.
The ATF6 arm of the UPR is activated when:
The ATF6 protein moves to the Golgi, where it is cleaved and its cytosolic domain enters the nucleus.