standard electrode potentials
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
describe an electrochemical cell:
2 half cells - electrodes submerged in solns of own ions
half cells connected by high resistance voltmeter and salt bridge

what is the purpose of the high resistance voltmeter?
measures p.d. between electrodes - this is the EMF/Ecell
typically e- would flow from more reactive to less reactive metal but high resistance inhibits e- flow to maintain conc of ions in soln
what is the salt bridge?
filter paper soaked in saturated soluble salt soln e.g. potassium nitrate
OR a tube containing unreactive ions in an agar gel
what is the purpose of a salt bridge?
helps to maintain electrical neutrality w/in internal circuit
allows movement of ions to complete the circuit and so prevent charge buildup ∴ prevents cell from taking its reaction to equilibrium
connects 2 ½ cells/electrodes but keeps solns separate
how does a salt bridge work?
to maintain electrical neutrality:
-vely charged ion moves to +ve ½ cell via salt bridge
+vely charged ion move to -ve ½ cell via salt bridge
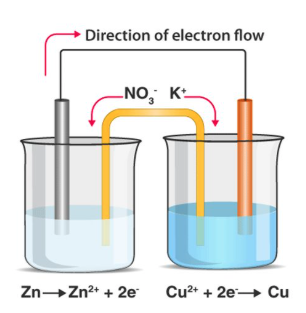
what happens when a piece of metal is submerged into a soln of its own ions?
an equilibrium is set up
there is a tendency for the metal to form +ve ions and go into soln
what happens if the PoE lies more to the left?
PoE lies more to the left:
metal acquires -ve charge due to e- buildup on metal
so electrode/half-cell has -ve potential
what happens when PoE lies more to the right?
PoE lies more to the right:
metal acquires +ve charge as e- have been used up to form metal from metal ions
so electrode/half cell has +ve potential
how does PoE change w/ reactivity?
more reactive metals - tend to form Mn+ ions ∴ -ve charge builds up on the metal
unreactive metals - less likely to form an ion ∴ +ve charge builds up on the metal
describe what would happen in a cell that has reached equilibrium (Zn and Cu cell):
using cell seen in diagram as an example:
e- flow externally in the circuit from the -ve ½ cell to the +ve ½ cell
Zn atoms in -ve ½ cell lose e- so are oxidised
e- flow from Zn metal to Cu metal
Cu2+ ions in soln gain e- to form Cu
Cu forms on the Cu electrode - mass of Cu electrode increases
some Zn metal forms Zn2+ ions - mass of Zn electrode decreases
∴ e- can flow
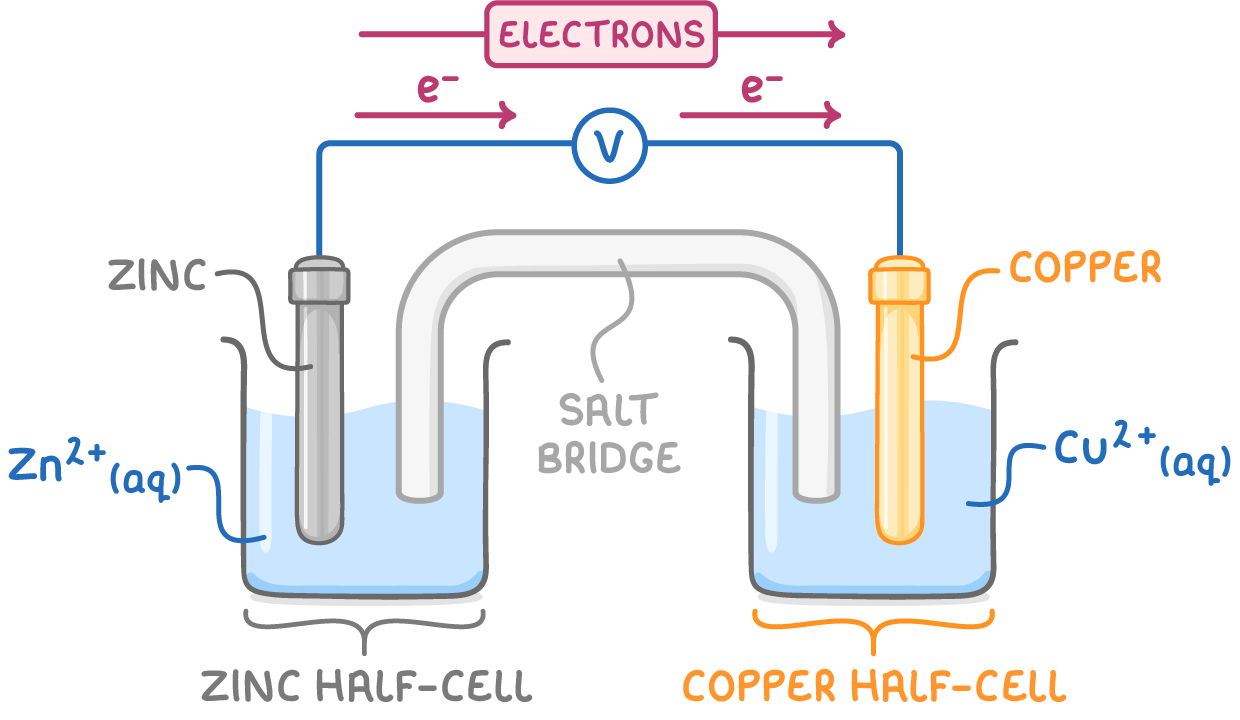
which side is the +ve electrode on (and so which side is the -ve electrode on?
+ve - RHS: more +ve Eθ
(-ve - LHS: more -ve Eθ)
at the +ve electrode, which reaction occurs? (oxidation/reduction)
reduction
so oxidation occurs at the -ve electrode