Development of the Atomic Model (History)
1/5
Earn XP
Description and Tags
With Years and Chronological Timeline
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
The Ancient Greeks
(490-460 BCE)
First recorded ideas about organization of matter —> came from ancient Greeks
Believed that all substances were made from four fundamental elements: water, fire, air, earth.
Greek Philosopher “Democritus” —> believed matter composed of tiny, indivisible particles called “atomos,” which is Greek for “indivisible.”
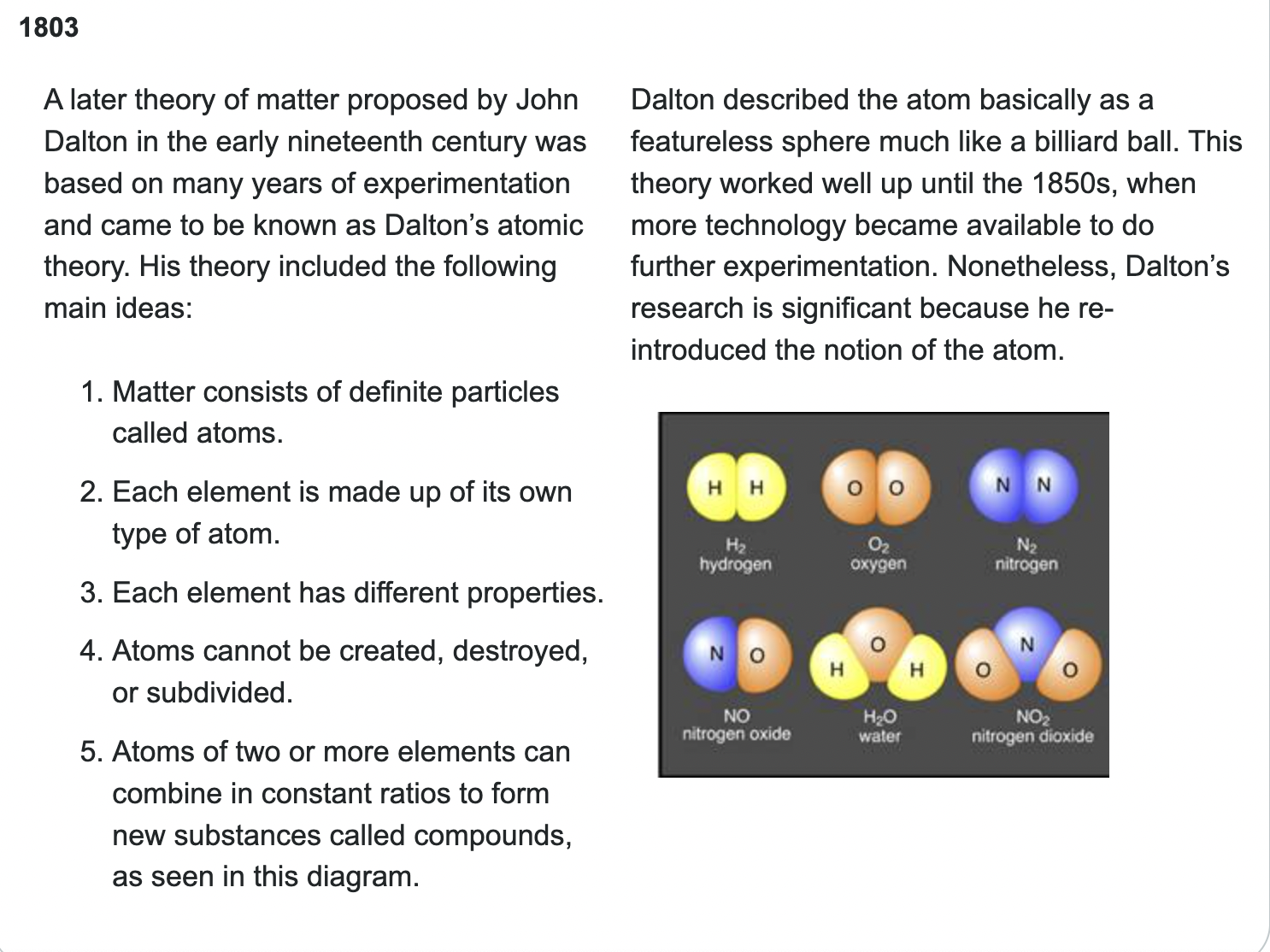
John Dalton
J. J. Thomson
(1893)
End of 19th century —> JJ Thomson awarded Nobel Peace Prize for forming a new idea about the atom
Used cathode ray tube —> discovered presence of particles within the atom
His model states: atom = positively charged sphere w/ negatively charged particles embedded w/in it
later named electrons
Model = analogous to chocolate chip cookie in which the:
Chips = negatively charged particles
Cookie dough = positively charged particles
Ernest Rutherford

Niels Bohr
1911
James Chadwick
1932
James Chadwick provided evidence for the neutron:
A dense, neutral (chargeless) particle located in the nucleus.
This completed the basic subatomic model of the atom:
Protons (positive charge)
Neutrons (no charge)
Electrons (negative charge, orbiting the nucleus)
Chadwick’s nuclear model set the stage for learning how electrons are organized in energy levels around the nucleus.