Chem Unit 1
1/128
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
129 Terms
Black body radiation
Light emitted by a perfect black object when heated
Study by Max Planck
Electromagnetic Spectrum
A range of electromagnetic waves
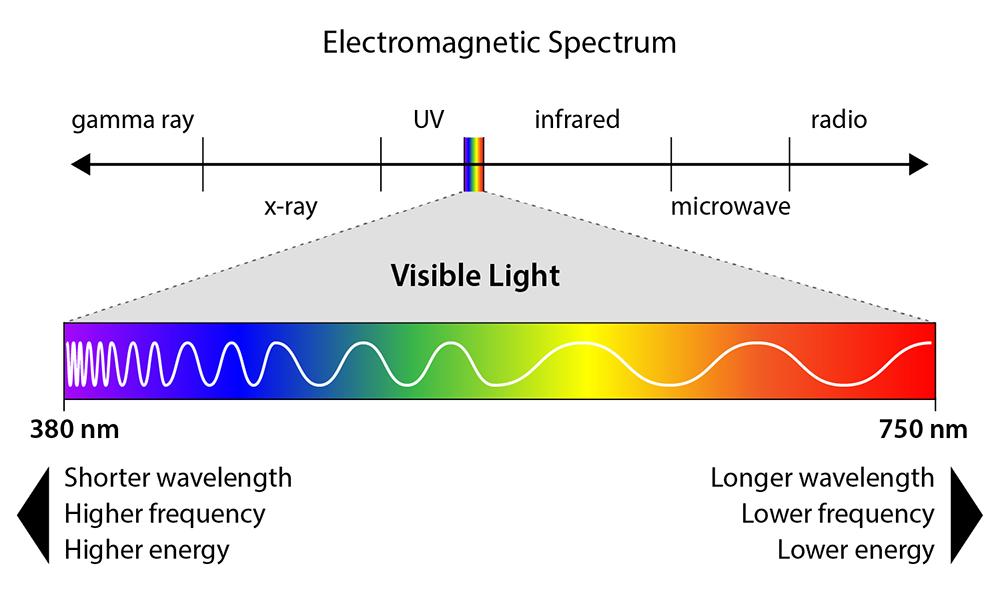
Wavelength
The length of a wave (λ)
Frequency
The number of cycles per unit of time (f)
Wave Equation
c = λf
λ = 3.0 × 10^8 m/s
f = s^-1 (Hz)
Photoelectric Effect
EM waves can give electrons enough energy to leave a substance. The shorter the wavelength, the greater the energy, therefore more electrons are able to leave.
Plancks equation
E = hf
E = energy of a quantum
h = Planck’s constant (6.63x10^-34)
f = frequency
Continuous Spectrum/Visible Spectrum
Splitting white light into its constituents using a prism (ROYGBV).
Bright Line Spectrum/Emission Spectrum
Shows specific quanta an element emits when heated up using a spectrophotometer.
Absorption Spectrum
Shows specific quanta an element absorbs when in cold gas form.
Bohr’s conclusions
Elements can only absorb and emit certain wavelengths of energy.
#1) Electrons do not emit energy when grounded
#2) Each orbit is an energy level - the further away the orbit, the higher the energy - electrons can jump to higher energy levels by absorbing more energy
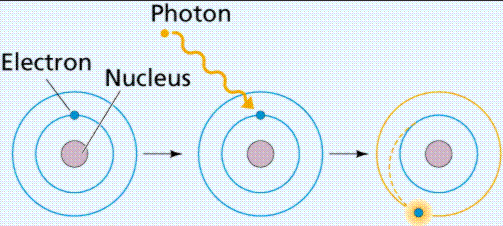
What is happening here?
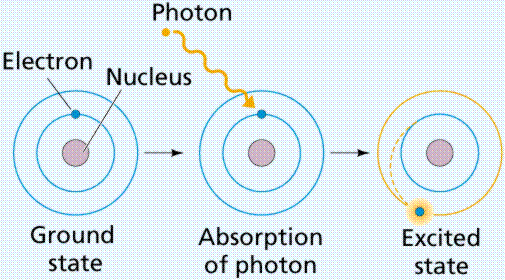
What did Balmer study?
He calculated the amount of photon energy released when electrons jump to ground state.
Balmer Series
Visible light - electrons jumps from any shell down to 2nd, producing visible light - violet is produced when there is a larger jump
Lynmann Series
(UV) - electron jumps down from any orbit to 1st
Paschen Series
(Infrared) - electron jumps down from any orbit to 3rd
How do you calculate the energy of an electron?
En = (-2.18x10^-18)/n2
n = shell number
How do you calculate the amount of energy released when an electron jumps down an orbit?
E = En - El
E = equivalent to E=hf
En = higher shell
El = lower shell
What formula relates wavelength and frequency?
c = λf
c = 3×10^8 m/s
What was Louis de Broglie’s Atomic theory?
Electrons do not move in circular orbits but in wave patterns
What was Heisenberg’s Atomic theory?
It is impossible to know the position and speed of an electron simultaneously.
What was Schrodinger’s Atomic theory?
He determined electron probability density (orbitals).
What are the main differences between the bohr model versus the quantum mechanical model?
Bohr Model: Protons and neutrons are in the nucleus, electrons orbit the nucleus in energy levels
Quantum Mechanical Model: Protons and neutrons in nucleus, electrons have translational movement around the nucleus in an energy level in a space called an orbital or probability density.
What is the Principal Quantum Number?
n = energy level
How do you determine the total number of:
a) orientations in an energy level
b) electrons in an energy level
a) n2
b) 2n2
What do each of these stand for?
1s2
1 = shell number
s = type of orbital
2 = number of electrons
What is the electron configuration for shell #3?
3s23p63d10
What is the electron configuration for shell #6?
6s26p66d106f14
What is the electron configuration for Na?
3s1
What is the electron configuration for Cr?
4s2 3d4
How do you draw orbitial diagrams for transitional metals?
1) remove electrons from s and p-orbitals first
2) don’t remove any electrons from d and f orbital if full
3) remove all electrons from s and p-orbitals all at once
How do you determine the following quantum numbers?
a) principal quantum number
b) second quantum number
c) magnetic quantum number
d) magnetic spin number
a) n = energy level/shell #
b) l = type of subshell orbital
s = 0, p = 1, d = 2, f = 3
c) ml = represents the number of orientations of each subshell
ml is a number from -l to +l
to determine, look at the position of the last electron in an orbital diagram
d) ms = the last electron in the orbital diagram
ms = +1/2 if unpaired
ms = -1/2 if paired
Intramolecular forces
The bonds between atoms in a molecule
Ionic Bonding
metal + non-metal
metal loses valence electron to become stable → + ions (cations)
non-metal gains valence electrons to become stable → - ions (anions)
What is the Lewis Dot Diagram for this Compound?
LiF
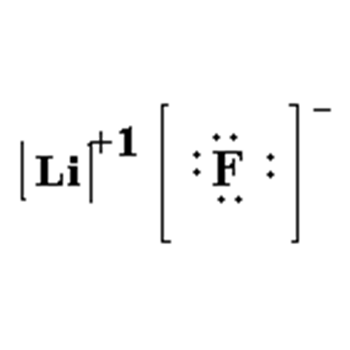
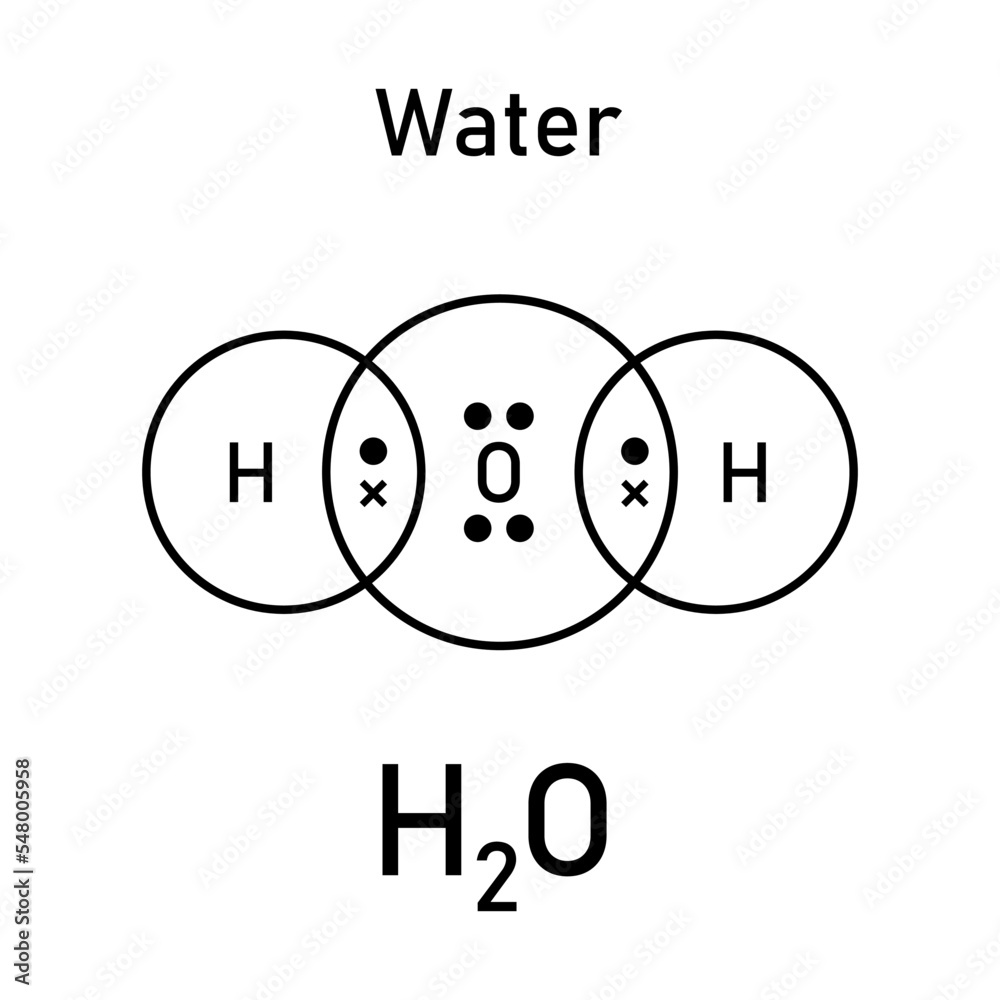
What is the Lewis Dot Diagram for H2O?
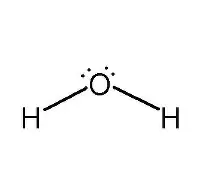
What is the Lewis Dot Structure for H2O?
Which is the center atom from the following molecules?
a) CH4
b) C2H3O2
c) H2O2
a) C - least number of atoms
b) C - can bond the most
c) O - can bond the most
What is a Co-ordinate Bond?
Also known as a Dative Bond, it is a bond where one atom shares both electrons.
One atom is full while the other requires 2 electrons to be full. Represented by an arrow that points to the atom that needs 2 electrons.
When is a co-ordinate bond not needed?
1) If the center atom is on the 3rd period or lower, they may have more than 8 electrons - they possess empty d-orbitals that allow them to take on more electrons. E.g. P and S
2) Certain center atoms can have less than 8 electrons. E.g. B and Be
How do you draw the Lewis structure for the following polyatomic ion?
H3O+
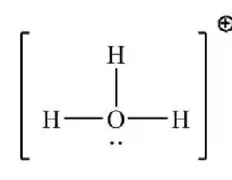
How do you draw the Lewis structure for the following polyatomic ion?
CO32-
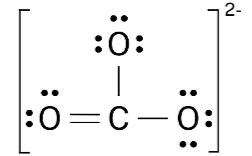
Resonance
Delocalized electrons in pi bonds can move around freely. This means that pi bonds can be switched around between atoms. E.g. NO3- has 2 resonance structures
What kind of bond are co-ordinate bonds?
Sigma bonds
Hybridization
A theory developed to explain the bonding and shape of molecular structures. It only occurs in molecules with center atoms.
Valence Bond Theory
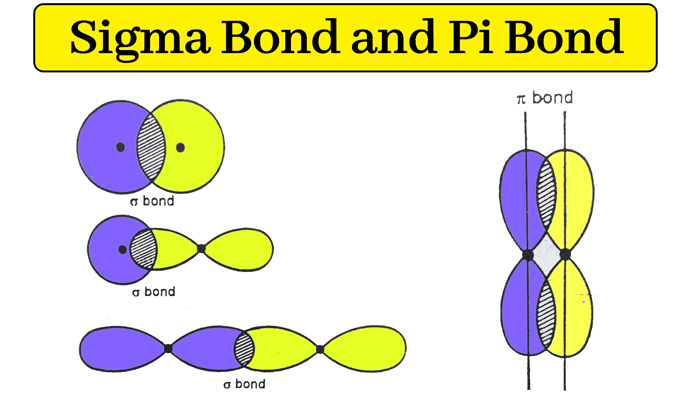
Covalent bonds share electrons between atoms as orbitals overlap. All covalent bonds have a sigma bond. In multiple bonds, one bond is a sigma bond and the extras are all pi bonds.
How are pi bonds created?
When unhybridized p-orbitals that are vertical to the sigma bond axial overlap.
Bond Length
The distance between the two nuclei of atoms in a bond. Multiple bonds have more electrons, therefore more electrostatic attraction of the electron to the nuclei, which shortens the bond length. The longer the bond length, the weaker the bond strength.
Bond Strength
The amount of energy needed to break a bond. Pi bonds have less bond strength than sigma bonds. Multiple bonds have more strength than single bonds. Therefore, bond strength is inversely proportionate to bond length.
Electron Domain
The number of lone pairs of center atom + the number of sigma bonds.
help determine the hybridized orientations
If the E.D. is 5, what is the hybridized orientations?
5 = sp3d
Hybridization
The process of combining orbitals to form new orbitals that are better suited for bonding and explaining molecular shapes. The energy level of the lower orbital gets promoted to an intermediate (balanced) level.
when asked for what is the hybridization, give the orbital combination e.g. sp²
Only occurs in compounds with a center atom
When hybridizing p-orbitals, why do you always show the left over orientations?
Because they form pi bonds.
V.S.P.E.R.
Valence Shell Pair Electron Repulsion
Who created VSPER and why?
Nyholm and Gillespie created this theory to explain molecular geometry
What are the 2 principles of VSPER?
1) Lone electron pairs and bonding pairs (e.g. sigma bonds) in the valence orbital repel each other (least to most repulsion: b.p. b.p. < b.p. l.p. < l.p. l.p.)
2) Lone pairs repel each other more than bonding pairs
Linear
B.p. = 2
L.p. = 0
+ any molecule that doesn’t have a center atom
Triangular Planar
B.p. = 3
L.p. = 0
V-Shape
B.p. = 2
L.p. = 1
VSPER - Double/Triple Bonds
Ignore pi bonds when using VSPER - they don’t affect the shape.
VSPER - More than 1 Center Atom
Look at each center atom individually. Treat as two separate molecules that are attached together.
Types of Intramolecular Forces
1) Ionic Bond (anion + cation) △EN = 1.7 - 4
2) Polar Covalent Bonding (electrons are unequally shared, and are closer to the atom with the greater △EN) △EN = 0.4 - 1.7
3) Pure Covalent (electrons are equally shared between atoms) △EN = 0 - 0.4
Electronegativity
An element’s ability to attract a bonding pair of electrons.
% Ionic Character
How much more ionic it is. The closer the △EN is to 4, the greater the ionic character.
Polar Molecules
Have an overall partial charge
Dipole Vectors
Arrows that represent the direction of the partial (-) charge. Can only draw dipoles if the bond between atoms is polar covalent.
How to determine if a molecule is Non-polar?
A molecule is non-polar if:
1) Center atom doesn’t have lone pairs (linear, triangular planar, tetrahedral, trigonal bipyramid, octahedral, square planar)
2) All bonds are the same
3) All bonds are pure covalent
When do Dipole vectors cancel out?
If the vectors face each other or away from each other. Doesn’t count if they are in an angle. Also doesn’t count if the △EN are different.
Intermolecular forces
Bonds between molecules
Ion-dipole
Occurs between an ion and a molecule with a dipole. Is the strongest IMF because there is a full charge
Hydrogen Bonding
Occurs with molecules that have both an H and F, O, or N. Second strongest IMF because FON have greatest EN.
Dipole-Dipole
The attraction between 2 polar molecules. 3rd strongest because charges are partial.
London Dispersion Force
Forces between non-polar molecules, formed from temporary dipoles as electrons move closer to one atom. The larger the molecule (greater MM), the larger the LDF because more electrons mean more attraction. Is the weakest because the charge is temporary.
Van der Waals force
The sum of all IMF forces in a molecule.
Surface Tension
Strength of molecules at the surface of a liquid (elastic skin)
Cohesion
Intermolecular forces between “same molecules”
Adhesion
Intermolecular forces between “unlike molecules”
Network Covalent Crystal (Giant Covalent/Giant Molecular)
Intramolecular force
substances made from several atoms that bond covalently in a regular repeated pattern
Usually formed with carbon and silicon
Allotropes (Different physical forms) of Carbon
1) Graphite - layers of hexagons of Carbon attracted by LDF, each carbon is only bonded 3 times, each carbon has a delocalized electron which moves from carbon to carbon, making graphite a conductor of electricity
2) Graphene - one layer of carbons in a hexagon, can conduct electricity because each carbon bonds 3 times, thus having a delocalized electron
3) Diamond - each carbon is bonded 4 times, can’t conduct electricity, tetrahedral
Metallic Bonding
Intramolecular force
the electrostatic attraction between a lattice of cations and delocalized electrons
electrons move from cation to cation, which is what allows metals to conduct electricity
cation layers can slide past each other, making metals malleable
Ionic Compounds
Intramolecular Force
the electrostatic attraction between cations and anions bonds the ion, forming an ionic lattice
the lattice is not fixed, meaning it simply follows the ration of cations to anions
Molecular Crystals
Intermolecular Forcees
solid molecular compounds which are attracted to each other by LDF in solid state
E.g. I2 (s)
Melting/Boiling Points
Determined by the strength of the intra and intermolecular forces, as they determine how much energy is needed to change states. Intra»Inter
Solubility
Substances dissolve in other substances when they are similar in polarity. “Like dissolves like”
1) Same polarity
2) Same IMF
3) Small molecule size
Conductivity
Something can conduct electricity if:
they have delocalized electrons e.g. metallic bonding, graphite, graphene
mobile ions e.g. aqueous or liquid ionic compound
Volatility
How easily a substance evaporates
stronger the IMF, the lower the volatility

Linear
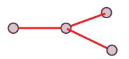
Trigonal Planar
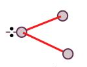
V-shape
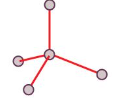
Tetrahedral
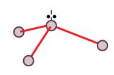
Trigonal Pyramid
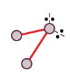
V-shape/angular
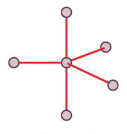
Trigonal Bipyramid
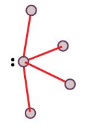
Seesaw
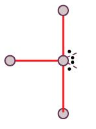
T-shape
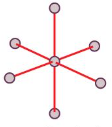
Octahedral
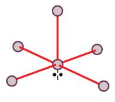
Square Pyramid
Square Planar
NH4+
Ammonium