Module 1: Transport of Small Molecules and Proteins
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
plasma membrane
defines the cell and separates the cytosol from the extracellular environment
membrane: phospholipid bilayer (2D fluid)
fluidity: olive oil like
noncovalent interactions between phospholipids, and between phospholipids and proteins provide membrane integrity and resilience
individual phospholipids spin and diffuse laterally within the plane of the membrane
barrier: hydrophobic core prevents unassisted movement of water-soluble substance from one side to the other
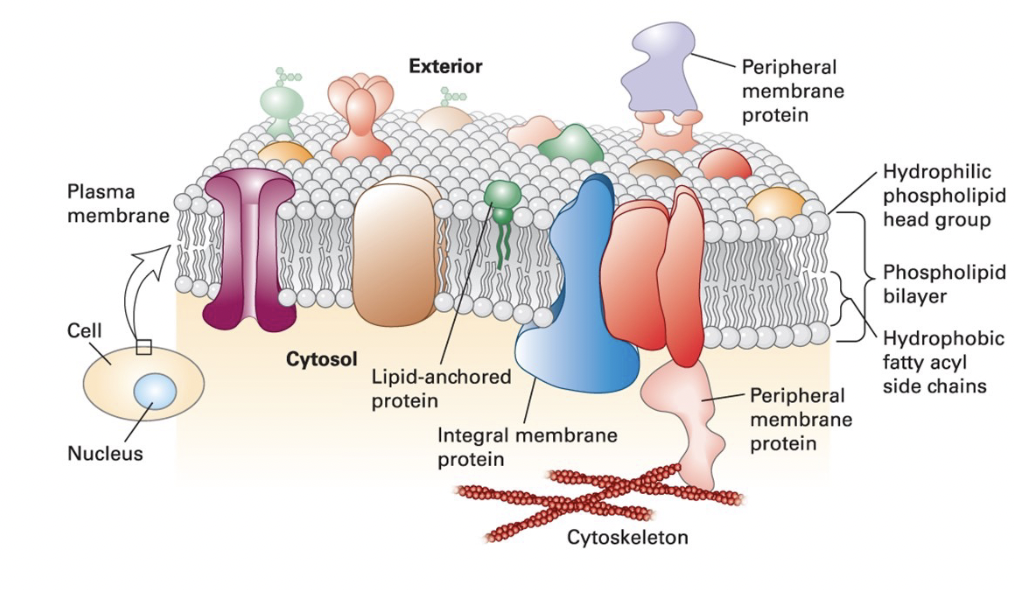
protein: membrane proteins provide each cellular membrane its unique set of function
integral membrane proteins (transmembrane proteins) span bilayer and often form dimer and high-order oligomers
lipid-anchored proteins tethered to one leaflet by a covalently attached hydrocarbon chain
peripheral protein associated primarily by specific noncovalent interactions with integral membrane proteins or membrane lipids
eukaryotic cellular membranes are dynamic structures
membrane fluidity and flexibility
enables organelles to assume their typical shapes
provides dynamic property that enables membrane budding and fusion
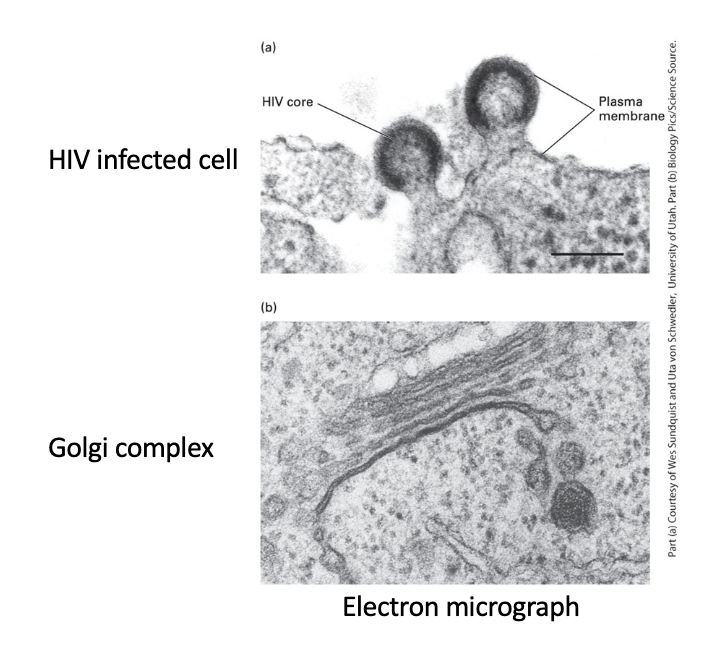
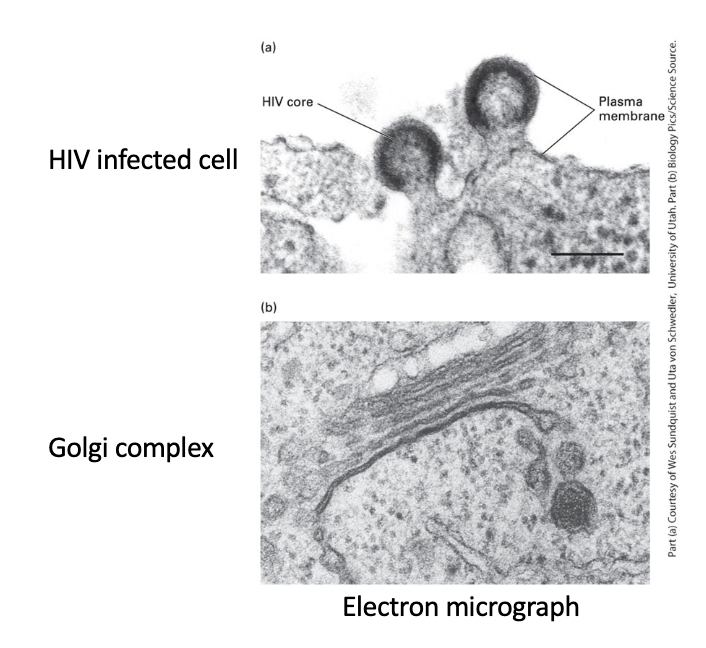
HIV-infected cell plasma membrane
virus core - enveloped by region of cell plasma membrane that contains specific viral proteins
HIV particles - bud from plasma membrane
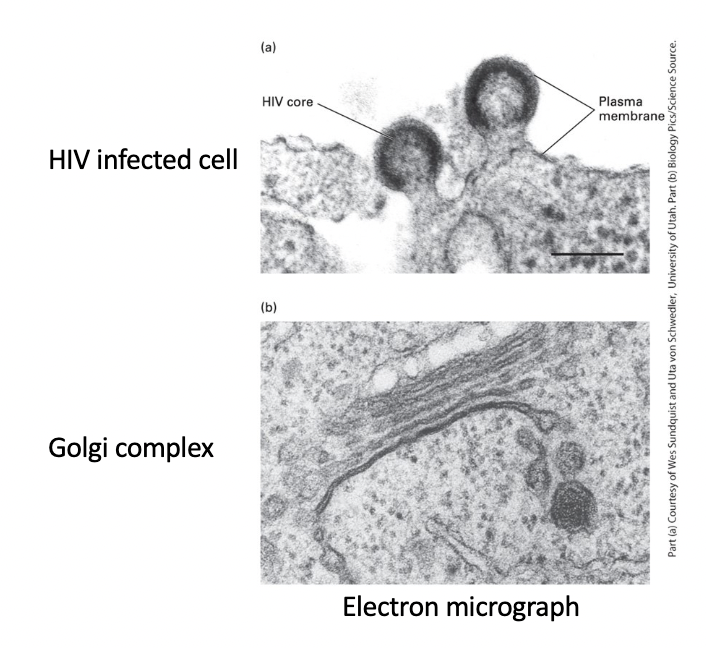
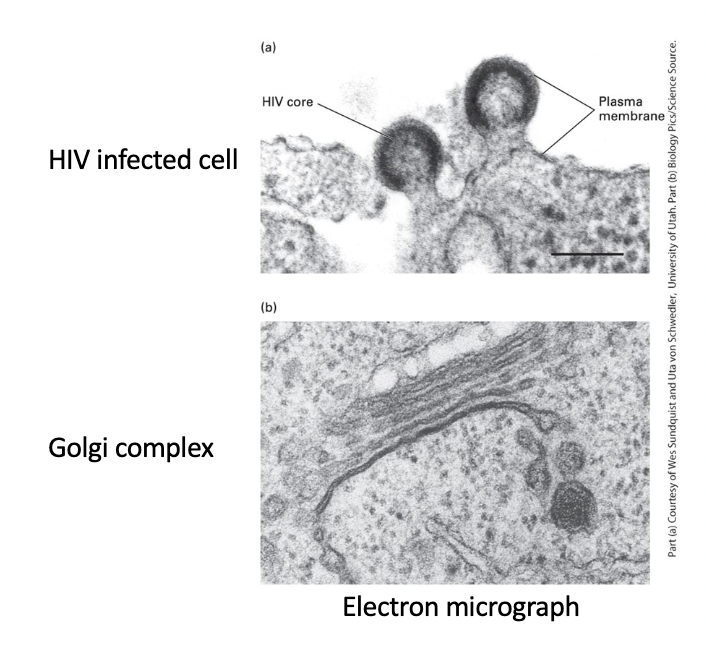
Golgi complex
stacked membranes with budding vesicles involved in intracellular trafficking
amphipathic
________ phospholipids spontaneously form bilayer with hydrophilic faces and a hydrophobic core
biological membranes
vary in lipid composition
impermeable to water-soluble molecules and ions
have viscous consistency with fluidlike properties
membrane phospholipids
amphipathic molecules - ends have different chemical properties:
hydrophobic fatty acid-based hydrocarbon “tail”
hydrophilic polar “head” which interacts with water molecules
erythrocyte membrane
stain interaction with hydrophilic heads and not hydrophobic tails yields characteristic “railroad track” appearance
true
T/F: Phospholipids structure form spontaneously, driven by behavior of hydrophilic and hydrophobic end exposure to water
nonpolar tails
close packing stabilized by van der Waals and hydrophobic effects interactions between the hydrocarbon chains
polar head groups
ionic and hydrogen bonds stabilize interactions with each other and with water
face outward to shield the hydrophobic fatty acyl tails from water
hydrophobic effect and van der Waals interactions between the fatty acyl tails drive the assembly of the bilayer
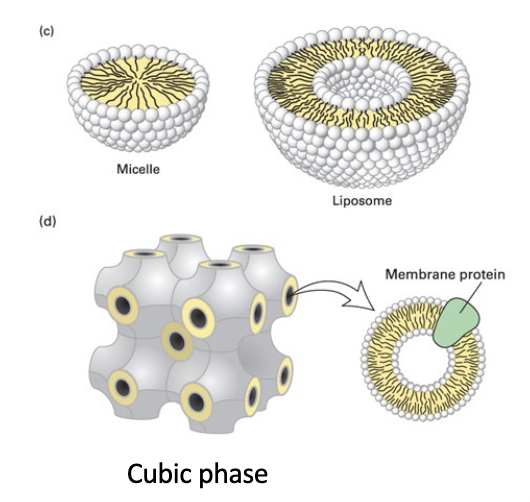
spherical micelle
phospholipid structure in water
hydrophobic interior composed entirely of fatty acyl chains
will not accommodate biomembrane phospholipids with 2 tails
detergents and soaps with less bulky tails form micelles
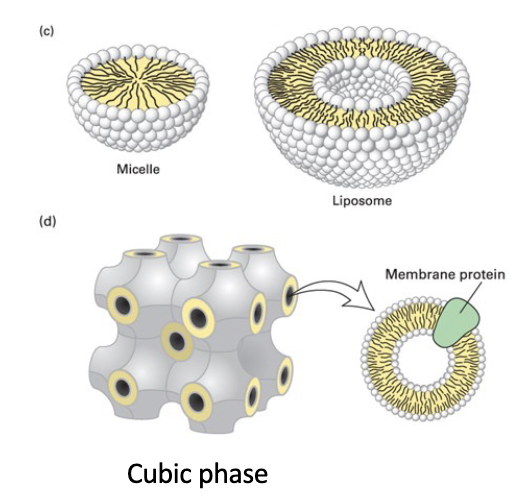
spherical liposome
phospholipid structure in water
phospholipid bilayer surrounding an aqueous compartment
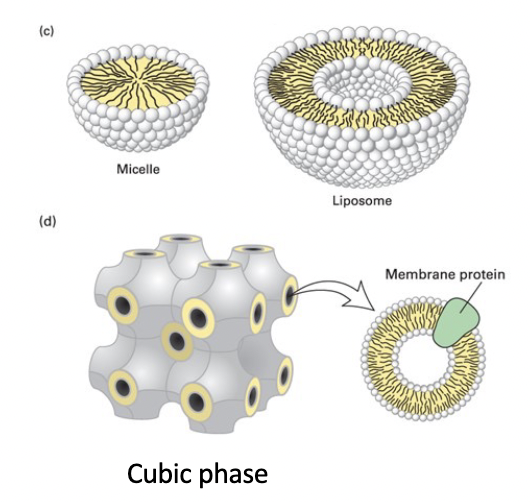
cubic phase
unnatural highly regular recurring structure
helped formation of membrane protein crystals for structure determination
true
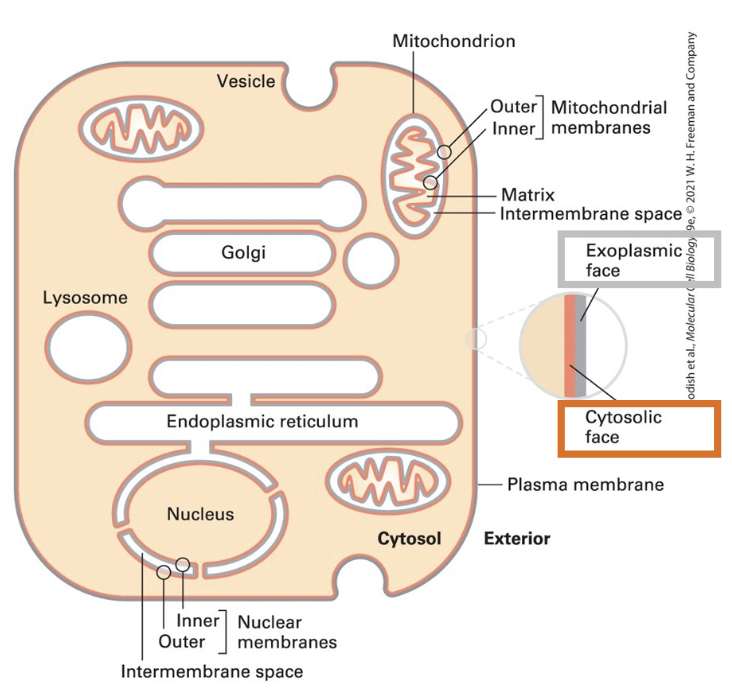
T/F: Cellular membranes have 2 faces, the cytosolic face and the exoplasmic face
true
T/F: The internal aqueous space is topologically equivalent to the outside of the cell
nucleus, mitochondrion, and chloroplast organelles
enclosed by two membranes separated by small intermembrane space
exoplasmic faces of the inner and outer membranes border the intermembrane space
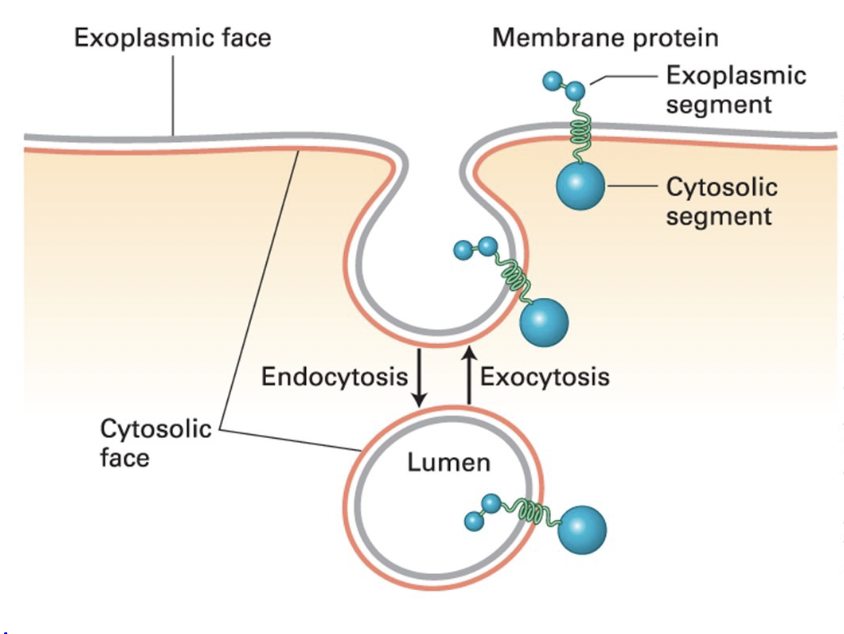
endocytosis
plasma membrane segment buds inward toward the cytosol and eventually pinches off a separate vesicle
cytosolic face - remains facing cytosol
exoplasmic face - faces vesicle lumen
exocytosis
an intracellular vesicle fuses with the plasma membrane
vesicle lumen connects with the extracellular medium
cytoplasmic face remains facing cytoplasm
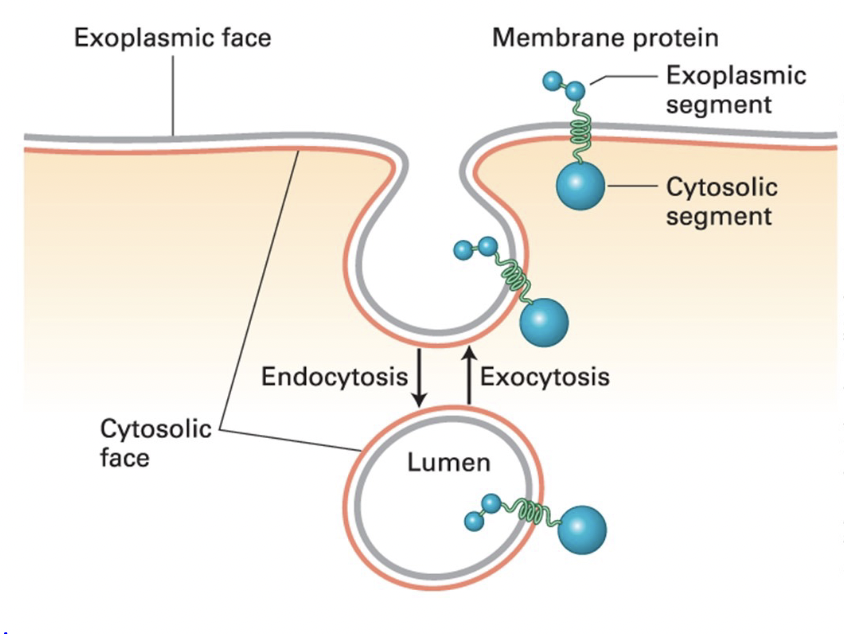
true
T/F: Membrane-spanning proteins retain asymmetric orientation during vesicle budding and fusion; same protein segment(s) always faces the cytosol
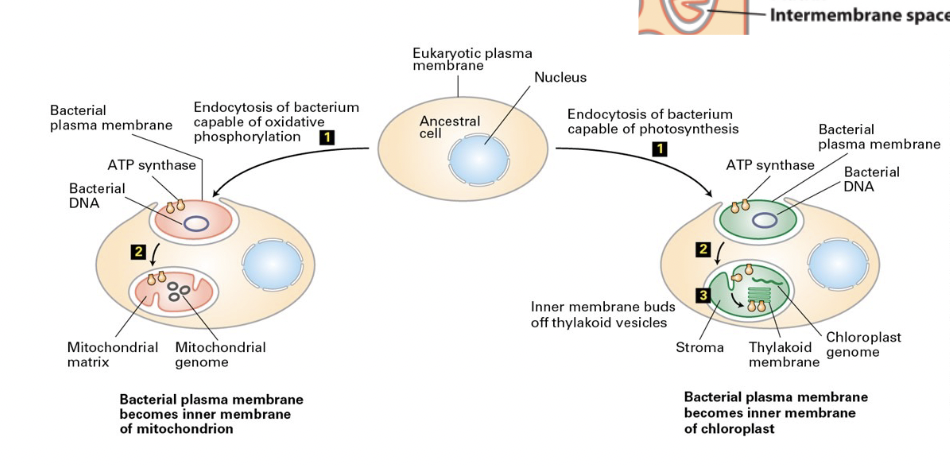
endosymbiont hypothesis
many lines of evidence that mitochondria and chloroplasts evolved from eubacteria engulfed into ancestral cells containing a eukaryotic nucleus
true
T/F: In organelles with two membranes the exoplasmic surface faces the space between the membranes
endocytosis of bacterium by an ancestral eukaryotic cell
1) eubacterium endocytosed with 2 membranes
2) becomes organelle with 2 membranes
outer membrane = derived from eukaryotic PM
inner membrane = originally the bacterial PM
inner membrane proteins would retain orientation
3) budding of vesicles from the inner chloroplast membrane vesicle; generates thylakoid membranes with the F1 subunit (ATP synthase) facing the chloroplast stoma
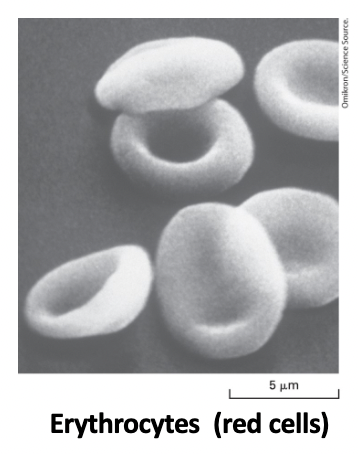
erythrocyte cell
discoid - flexible shape required for squeezing through blood capillaries with smaller diameters
shape defects cause cell lysis (anemias)
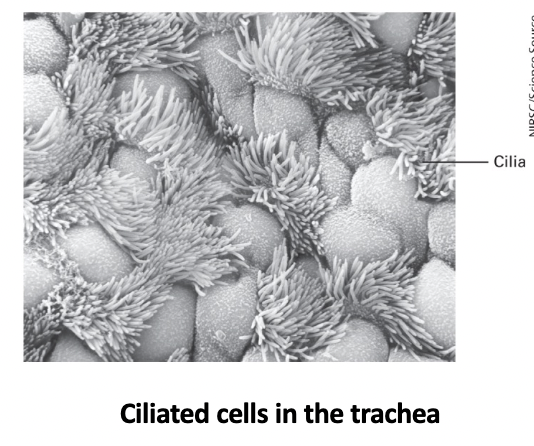
ciliated cells in the trachea
cilia extensions of the PM contain microtubules and motor proteins that enable them to produce patterns of shape changes that move materials across epithelial surfaces or propel cell motility
an immotile primary cilium plays key roles in cell signaling
three classes of lipids
differ in structure, abundance, and function
phosphoglycerides (phospholipids)
sphingolipids (phospholipids or glycolipids)
sterols
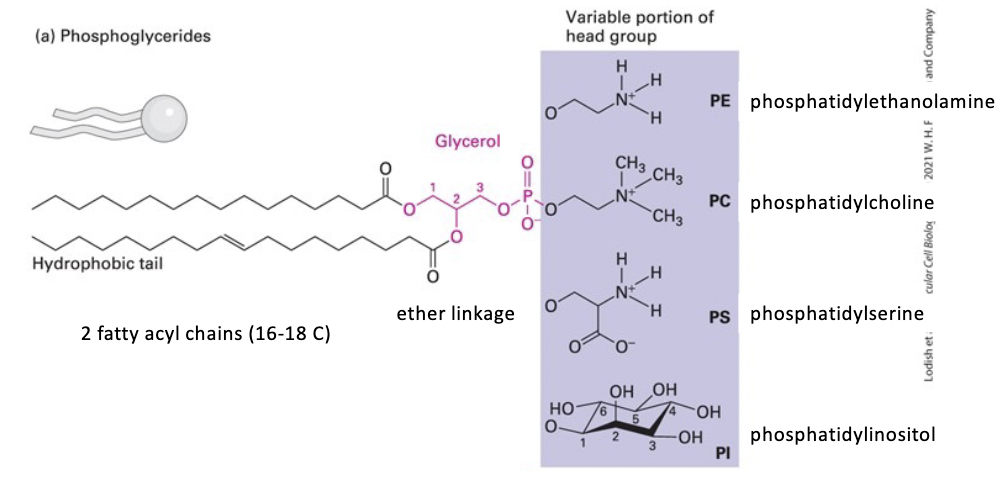
phosphoglycerides
most abundant in biomembrane
glycerol backbone
tails - 2 esterified hydrophobic fatty acyl chains
usually 16C-18C
vary in saturation (saturated/unsaturated or bonds/double bonds)
head - a polar group esterified to the phosphate (4 types)
phosphatidylcholine (PC)
phosphatidylethanolamine (PE)
phosphatidylserine (PS)
phosphatidylinositol (PI)
sphingolipids
derivatives of sphingosine (an amino alcohol with a long hydrocarbon chain)
various fatty acyl chains connected by an amide bond
some are glycolipids that contain a single sugar residue or branched oligosaccharide attached to the sphingosine backbone (ex: Glucosylcerebroside GlcCer has a glucose head group)
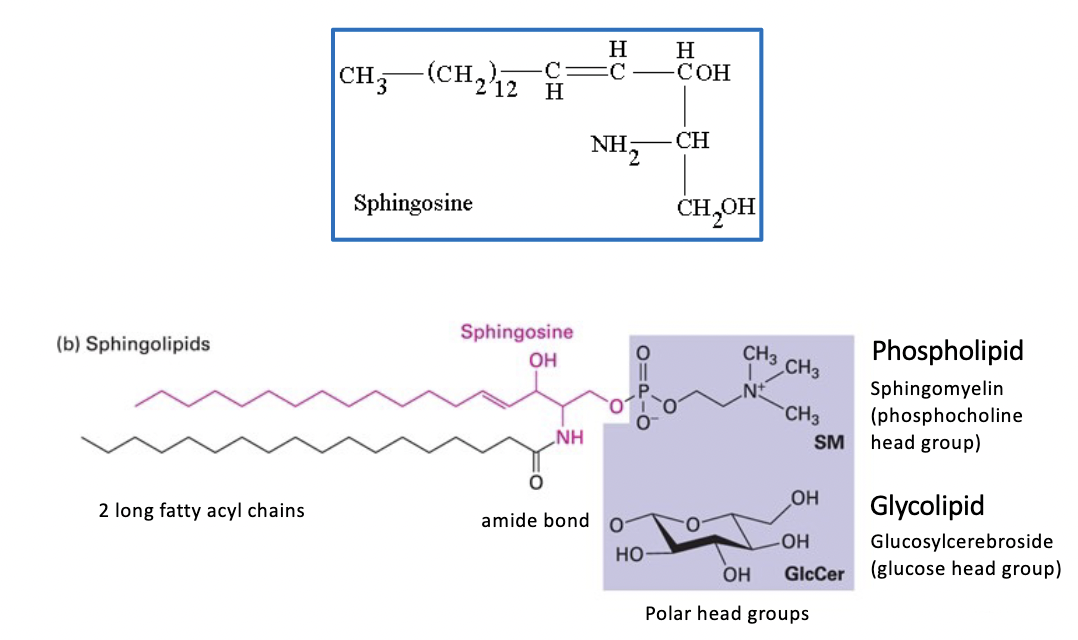
sphingomyelins (SM)
contain a phosphocholine head group
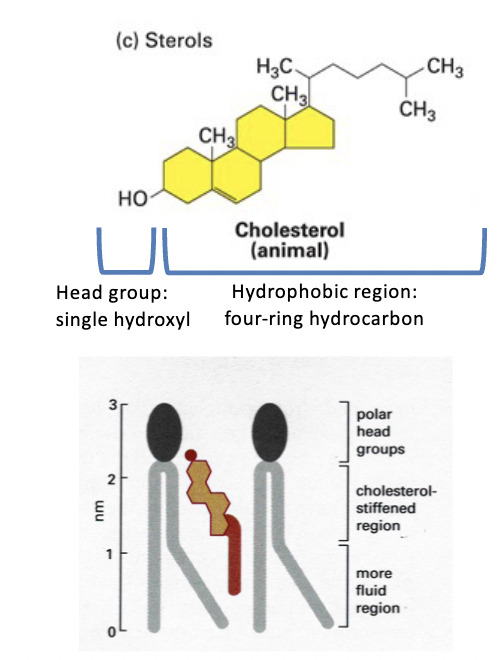
sterols
membrane components - animals (cholesterol), fungi (ergosterol), and plants (stigmasterol)
amphipathic structure
head group = single polar -OH
tail = conjugated four-ring hydrocarbon and short hydrocarbon chain
very hydrophobic
cannot form bilayers on its own, but it intercalates into biomembrane
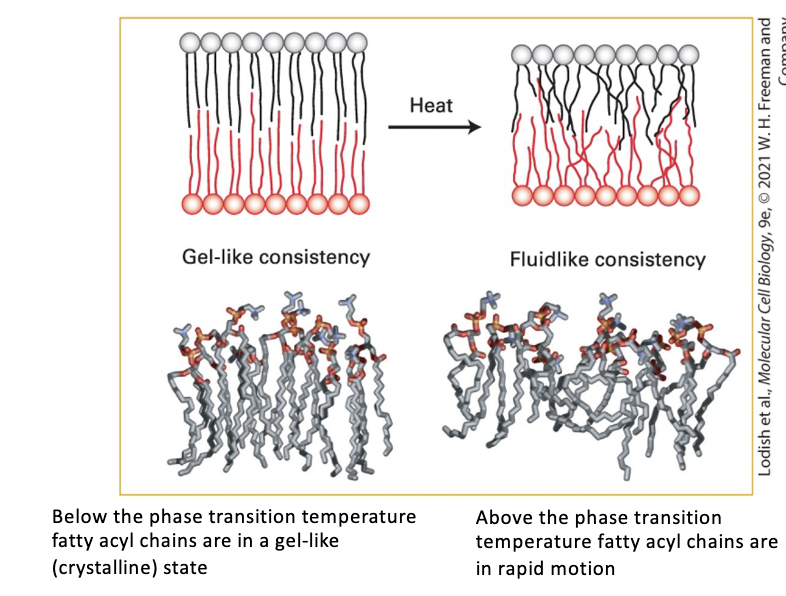
temperature influence on biomembrane
COLD
gel-like consistency
below the phase transition temperature fatty acyl chains are in a gel-like (crystalline) state
HEAT
fluid-like consistency
above the phase transition temperature fatty acyl chains are in rapid motion
heat disorders nonpolar tails induces a transition from a gel to a fluid within a temperature range of only a few degrees
chain disorder increases bilayer thickness
lipid composition
within same cell, different membranes have different lipid compositions
ex: Golgi membranes contain more sphingomyelin than ER membranes
different types of cells have membranes with different _________
ex: PM of intestinal cells contains more sphingolipids and less phosphoglycerides then most
fluid
A more _______ state is favored by lipids with short fatty acyl chains (like phosphoglycerides
gel-like
A more _______ state is favored by longer more saturated fatty acyl chains that pack tightly together (like sphingolipids)
cholesterol
regulates membrane fluidity during normal cell growth and restricts random movement of phospholipid head groups at the outer surfaces of the leaflets
it’s steroid ring interaction with the long hydrophobic tails of phospholipids immobilizes lipids and decreases biomembrane fluidity
decreased
In general, membrane fluidity is ___________ by sphingolipids and cholesterol and increased by phosphoglycerides.
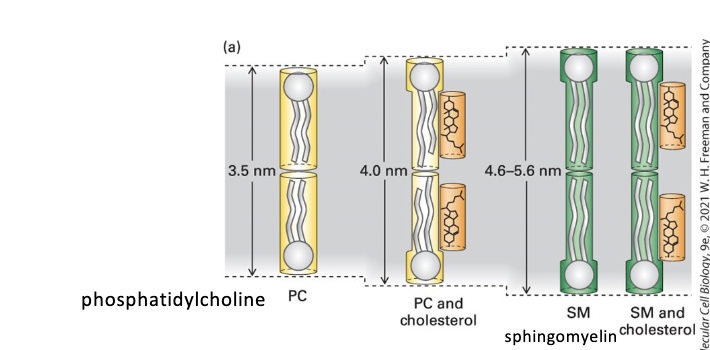
membrane thickness
PC < PC + cholesterol < SM < SM + cholesterol
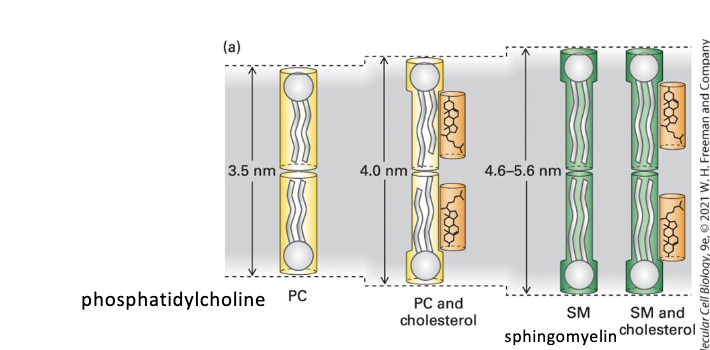
pure sphingomyelin (SM) bilayer
thicker than phosphoglycerides (phosphatidylcholine) bilayer
cholesterol lipid-ordering effect increases phosphoglyceride bilayer thickness
lipid rafts are thicker than other membrane regions
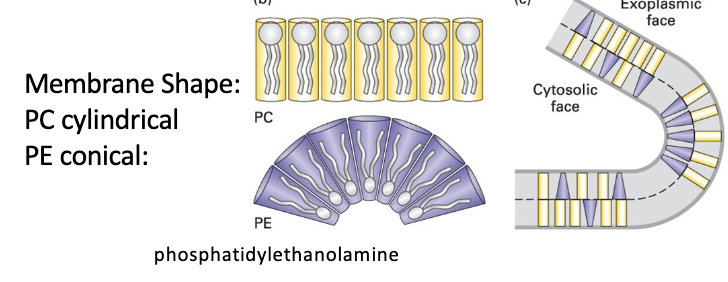
membrane curvature
PC cylindrical shape - forms essentially flat monolayers
PE conical shape (smaller head group) - forms curved monolayers
lipid rafts
microdomains containing cholesterol, sphingolipids, and certain membrane proteins that form in the plane of the bilayer; these lipid-protein aggregates regulate signaling by certain plasma membrane receptors
thicker than most bilayers
enriched in glycoplipids
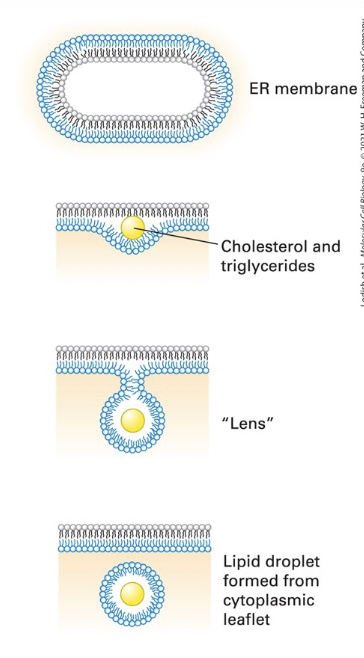
lipid droplets
storage compartments for triglycerides and cholesterol esters, also may serve as platforms for storage of proteins targeted for degradation
formation:
cholesterol esters and triglycerides accumulate within the hydrophobic core of the lipid bilayer
delamination of the two lipid monolayers forms a “lens”
lens growth creates a spherical droplet released by scission at the neck
the newly formed droplet is surrounded by a lipid monolayer derived from the cytosolic leaflet of the ER membrane
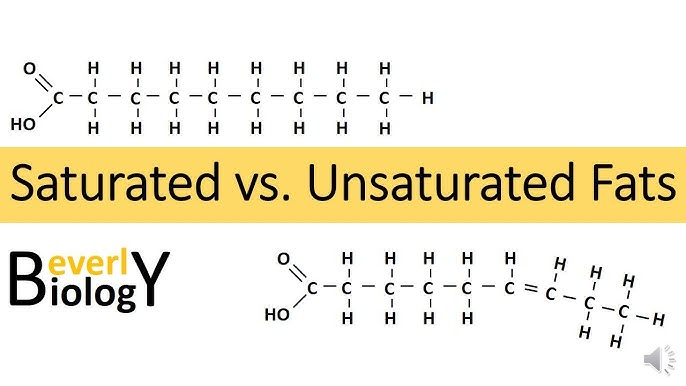
saturated fats
type of fat containing high proportion of fatty acid molecules WITHOUT double bonds, considered to be less healthy in the diet
ex: butter
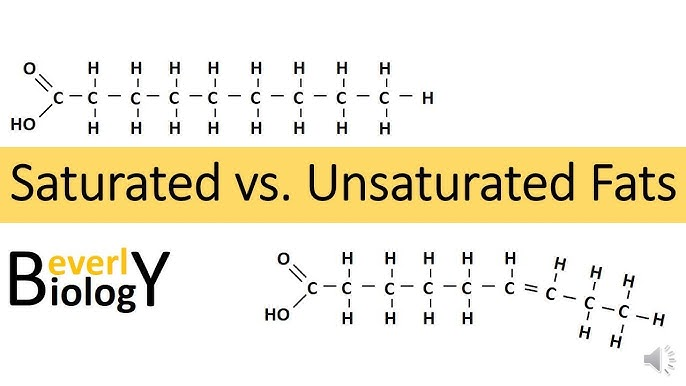
unsaturated fats
healthy dietary fats characterized by having one or more double bonds in their fatty acid chains, making them liquid at room temperature
ex: olive oil
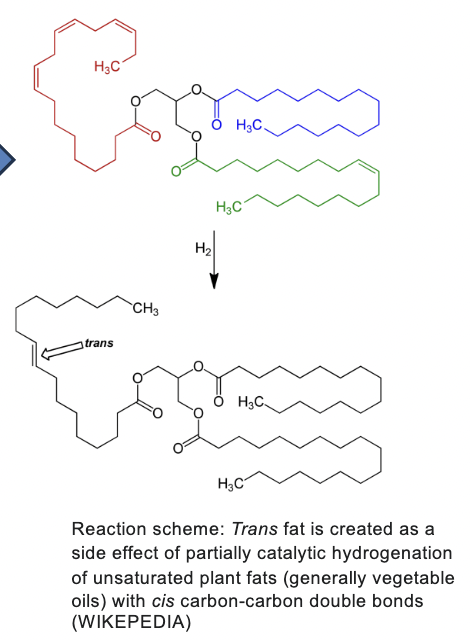
trans fat
created as a side effect of partially catalytic hydrogenation of unsaturated plant fats (generally vegetable oils) with cis carbon-carbon double bonds
membrane-spanning domain
Integral membrane proteins contain one or more hydrophobic ___________
asymmetrically oriented
Transmembrane proteins and glycolipids are ___________ in the bilayer
asymmetry
typical single-pass transmembrane protein → glycophorin A
glycosylation occurs solely on the exoplasmic side
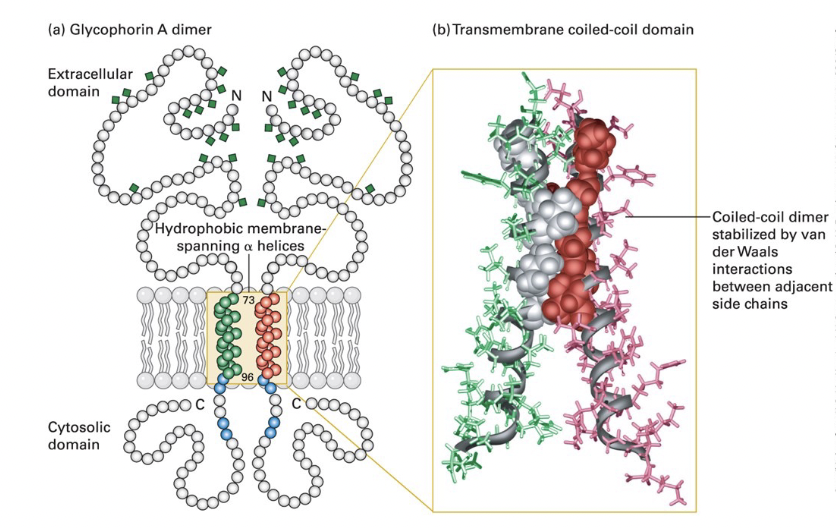
dimeric glycophorin
single (23-residue) membrane-spanning α helix
composed of amino acids with hydrophobic (uncharged) side chains
α helix typically 20-25 AA long in transmembrane proteins
positively charged arginine and lysine residues near the cytosolic side of the helix bind negatively charged phospholipid head groups to anchor glycophorin in the membrane
extracellular domain - heavily glycosylated; carbohydrate chains attached to specific serine, threonine, and asparagine residues
cytosolic domain - interacts with cytoskeletal proteins
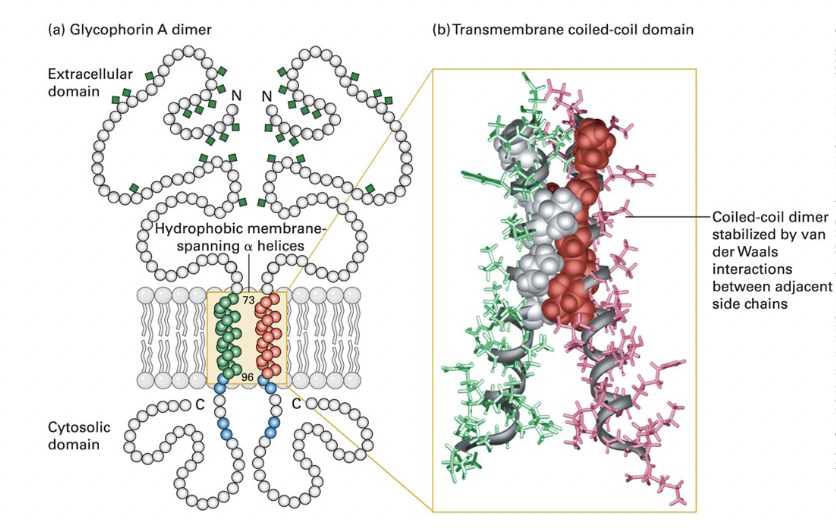
transmembrane domain
hydrophobic side chains of the α helix interact with surrounding membrane lipids
coiled-coil dimer interface; hydrophobic side chain van der Waals interactions between several AA
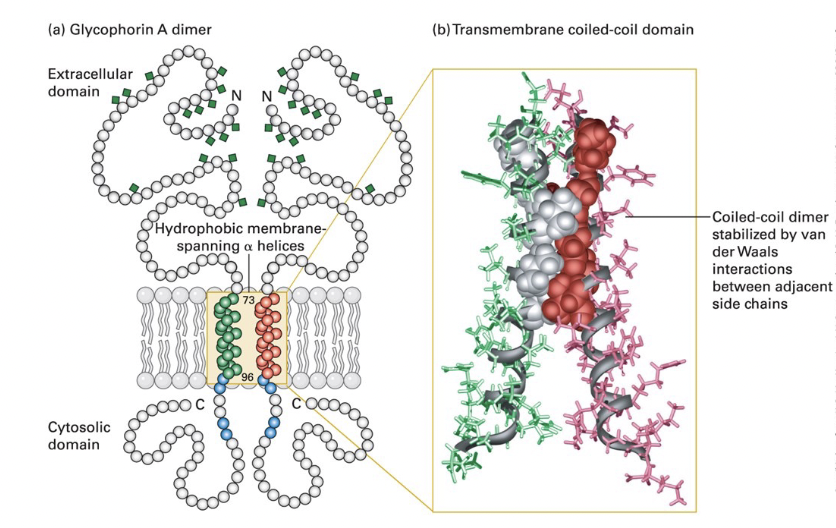
charged residues
polar or __________ in α-helical transmembrane segments can guide assembly an d stabilization of multimeric membrane proteins
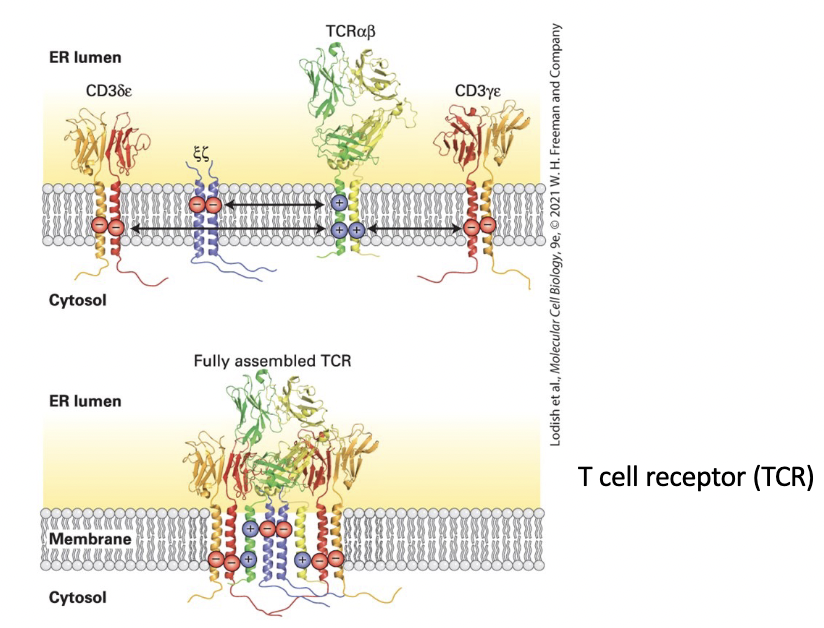
T cell receptor (TCR) for antigen
composed of 4 separate dimers:
αβ pair directly responsible for antigen recognition
CD3 complex accessory subunits – γ, δ, ε, and ζ subunits
Electrostatic attraction of positive and negative charges on each transmembrane domain forms the complete complex.
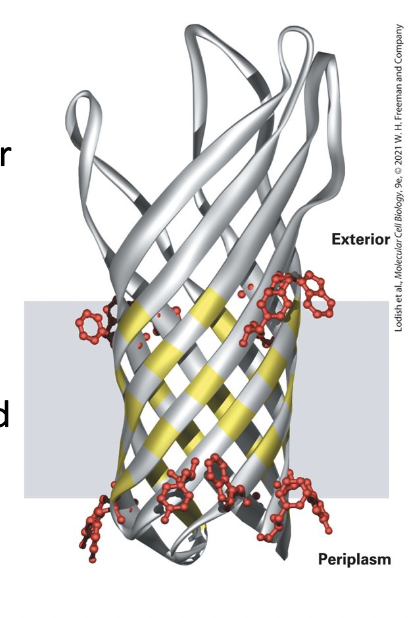
porins
pore-forming proteins that span the bilayer as a β-barrel
barrel shaped unit
alternating outward-facing hydrophobic side chains on each β strand position the protein in the bilayer.
alternating inward-facing hydrophilic side chains line the pore.
β strands form the wall around a water-filled transmembrane pore in the center, through which small hydrophilic can diffuse.
16 antiparallel β-sheets
hydrophobic side chains exposed to the bilayer
hydrophobic residues exposed to pore
anchoring
covalently attached lipids anchor some otherwise water-soluble proteins to one or the other plasma membrane leaflet in eukaryotic cells
acylation
cytosolic proteins (such as v-Src) anchored to the PM through a single fatty acyl chain attached to N-terminal Gly
common acyl anchors → myristate (C14) and palmitate (C16)
v-Src: a viral mutant form of cellular tyrosine kinase, induces abnormal cellular growth that can lead to cancer when anchored to the membrane by myristylation
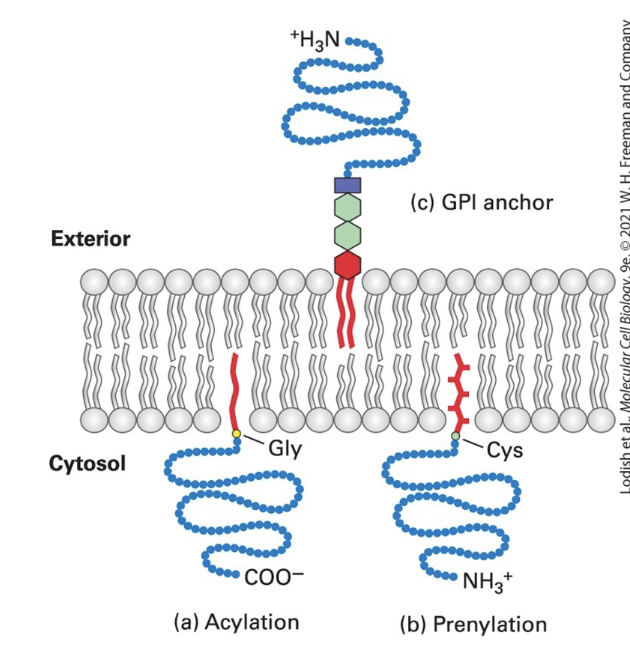
prenylation
cytosolic proteins (such as Ras and Rab G-proteins) anchored to the membrane through prenyl group thioether bond to one or two C-terminus Cys-SH groups
CAAX box Cys prenylated and AAX removed)
common anchors → unsaturated farensyl (C15) and greanylgeranyl (C20) groups
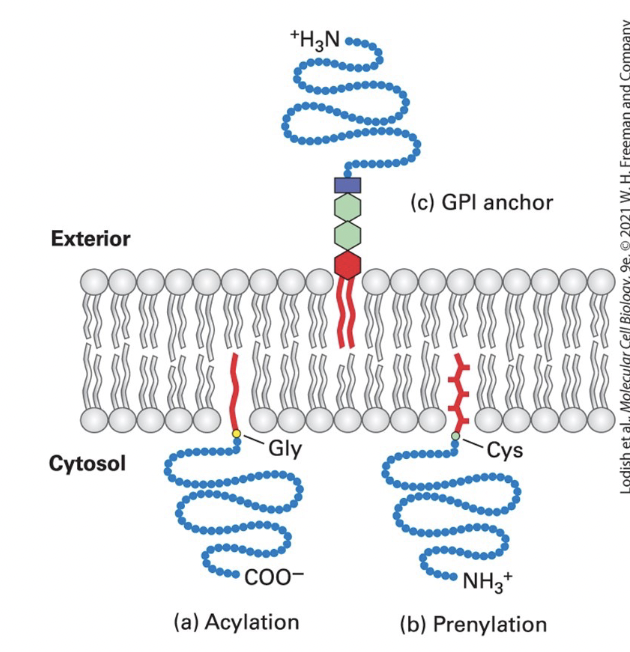
GPI (glycosylphosphatidylinositol) lipid anchor
anchors extracellular protein to exoplasmic surface of the PM
phosphatidylinositol anchor → contains 2 fatty acyl chains inserted into bilayer
phosphoethanolamine unit → links protein to the anchor
sugar units → vary in number, nature, and arrangement in different anchors
can cluster in lipid rafts
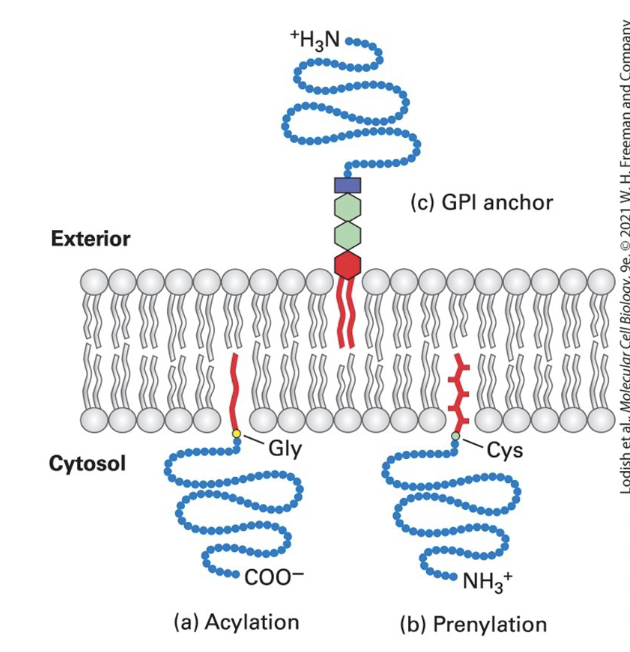
ABO blood types
3 structurally related oligosaccharides components of certain glycoproteins and glycolipids on the surface of human RBC and other cells
the terminal oligosaccharide sugars distinguish the O, A, B, and AB antigens
presence or absence of glycotransferases that add galactose (Gal), N-acetylgalactosamine (GalNAc), or both to the O antigen determines a person’s blood type
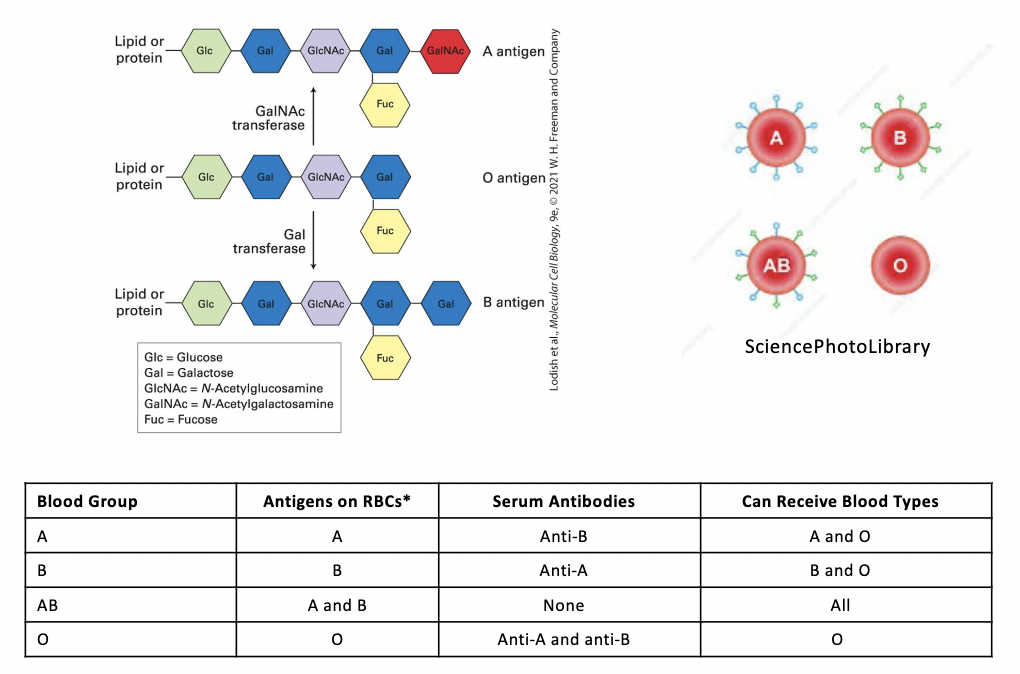
phospholipase A2
structure - lipid-binding rim of positively charged arginine and lysine residues surrounds the catalytic active site cavity
catalysis - positively charged binding site rim residues bind to negatively charged polar groups at membrane surface
small conformational change opens a channel to catalytic site lined with hydrophobic AAs
phospholipid moves from the membrane leaflet into the channel
enzyme-bound Ca ion binds the lipid head group, positions ester bond to be cleaved int he catalytic site
phospholipase
enzyme activity - each type hydrolyzes a specific bond in a phospholipid
functions:
degrade damaged/aged cellular membranes
generate signaling molecules
contribute to destruction caused by many snake venoms
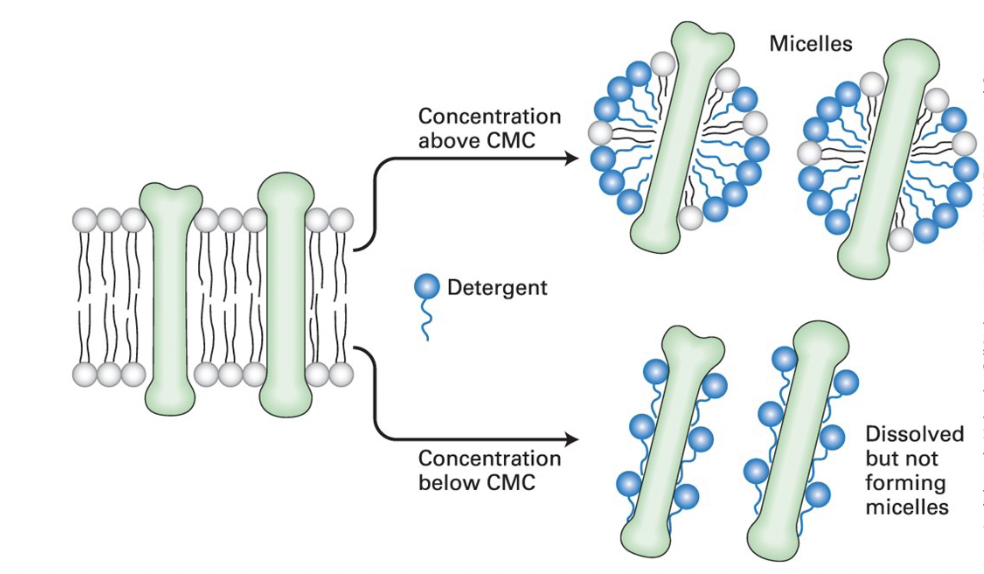
critical micelle concentration (CMC)
detergent concentration at which micelles (soapy bubbles) form
concentration higher than CMC → detergent solubilizes lipids and integral membrane proteins, forming mixed micelles containing detergent, protein, and lipid molecules
concentrations lower than CMC → non-ionic detergents dissolve membrane proteins without forming micelles by coating the protein membrane-spanning regions
ER
Fatty acids are synthesized in the _____ and moved to other membranes by multiple mechanisms
flippases
Phospholipids are asymmetrically distributed in the bilayer due to the action of _________
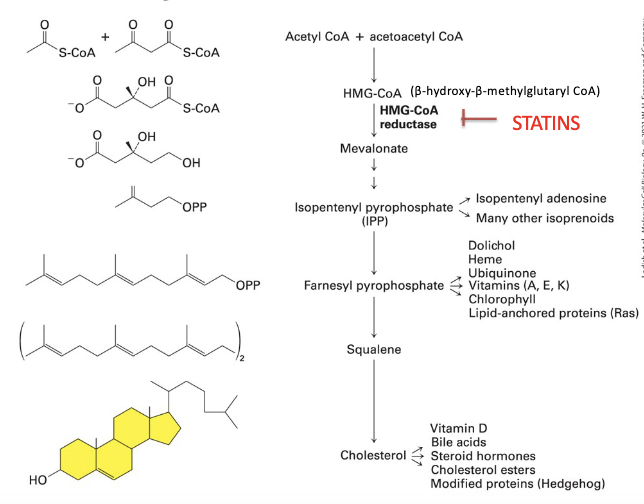
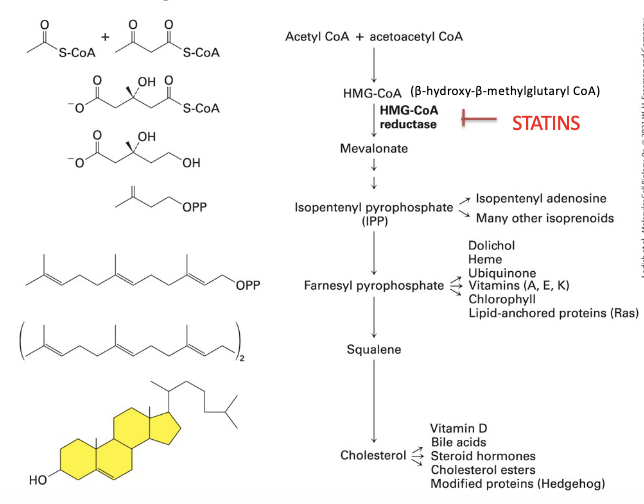
HMG-CoA reductase
__________ catalyzes the cholesterol biosynthesis rate-controlling step
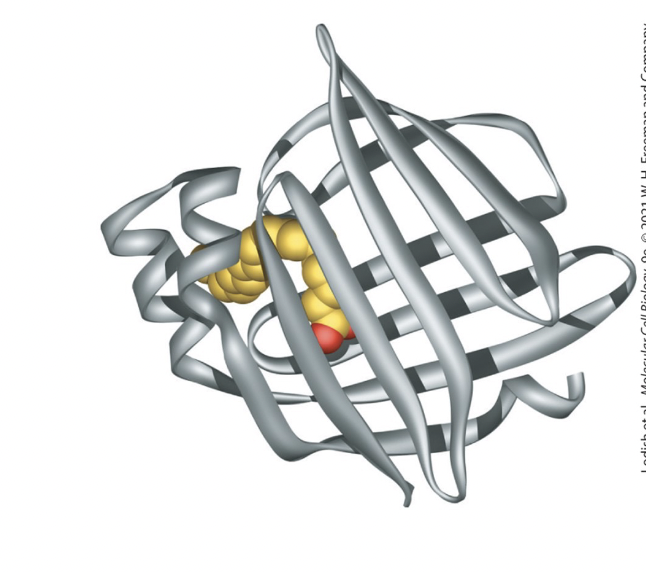
fatty acid-binding protein (FABP)
small cytosolic proteins facilitate movement of fatty acids
contain a hydrophobic pocket lined by β sheets that binds fatty acids
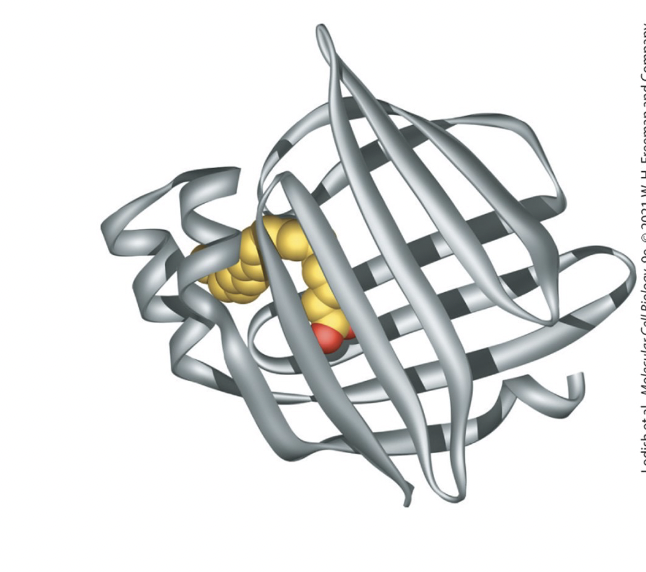
adipocyte FABP
has 2 β sheets, at right angles to each other, forming a clam shell structure
fatty acid interacts noncovalently with hydrophobic AA residues within this pocket
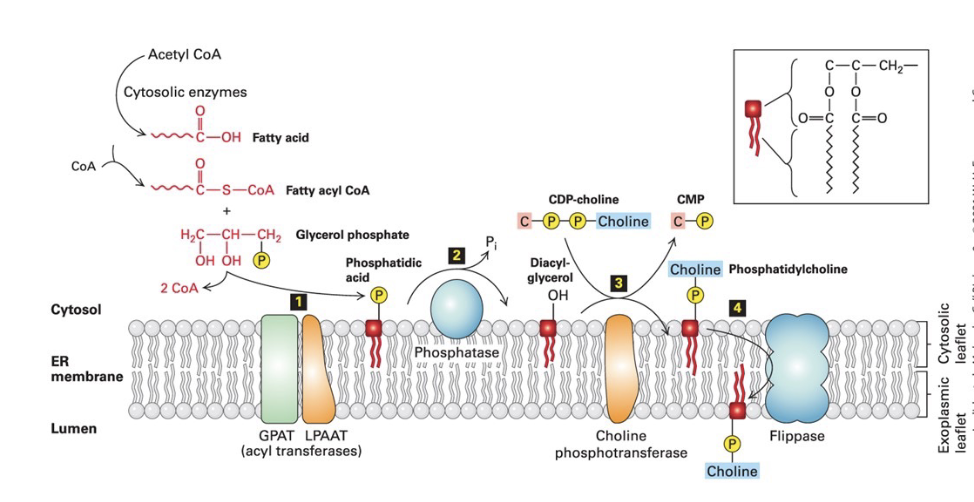
phospholipid synthesis in ER membrane
Step 1)
2 fatty acids synthesized on fatty acyl CoA - esterified to the phosphorylated glycerol backbone, forming a phosphatidic acid
hydrocarbon tails anchor the molecule to the membrane
Step 2) phosphatase → converts phosphatidic acid into diacylglycerol (DAG)
Step 3) phosphotransferase transfers polar head group (ex: phosphorylcholine) from CDP-choline to the exposed OH group to make phosphatidylcholine
Step 4) flippase → uses ATP to catalyze movement of phospholipids from cytosolic leaflet to the exoplasmic leaflet to equalize leaflet growth and establish asymmetry
cholesterol biosynthetic pathway
1) HMG-CoA reductase (RATE CONTROLLING STEP)
converts β-hydroxy-β-methylglutaryl CoA (HMG-CoA) to mevalonate
2) mevalonate → converted into IPP
3) IPP → converted into cholesterol and other lipids, through polyisoprenoid intermediate
cholesterol regulation
when there is high cholesterol levels in the ER membrane
cholesterol binds to HMG-CoA reductase sterol-sensing domain
causes interaction with integral ER membrane proteins (Insig-1 and Insig-2) which induces ubiquitnylation and degradation of HMG-CoA reductase by a proteasome
reduces production of mevalonate, the key intermediate in cholesterol biosynthesis