Synaptic Transmission and Neurotransmitters
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
Define a synapse
The junction between two neurons where information is transmitted from the axons of one to the dendrites of another
Define Electrical Synapse
The electrical current travels from the presynaptic cell to the postsynaptic cell through a gap junction.
Define Chemical synapse
A neurotransmitter is released from the presynaptic neuron into the synaptic cleft and binds to a receptor on the postsynaptic neuron causing a response
What will decrease the effect of a neurotransmitter both Presynaptic (2) and postsynaptic (2)
Presynaptic:
Block voltage-gated Ca2+ channels
Something prevents the release of the neurotransmitter
Post synaptic
Something causes a reduced number of receptors on the postsynaptic membrane
Something decreases the activity of the receptor so there is less of an effect when the neurotransmitter binds to the receptor
Summary of Neurotransmitter Release (4)
Membrane depolarization opens
voltage gated Ca++ channels causing
Ca++ influx
The increase in intracellular Ca++
increases the binding of Ca++ to
synaptotagmins
This triggers a conformational change
in the SNARE complex (Before the
action potential, vesicles are loosely
docked by SNARE proteins)
The change in SNARE proteins
causes vesicle membranes to fuse
with the plasma membrane and
neurotransmitter release
What will increase (2) and decrease (2) the release of a neurotransmitter Presynaptically
Presynaptic
Increase the Ca2+ influx by enhancing the activity of voltage-gated Ca2+ channels
Something causes a greater increase in intracellular Ca++
Postsynaptic
Something decreases the activity or causes the degradation of synaptotagmins or SNARE proteins
Something prevents the increase in intracellular Ca++
The ventricles in the heart act as a functional
syncytium (i.e. contract as one unit because
the muscle cells contract synchronously). In
order for this to happen, the signal to
contract (which are action potentials) goes
from one cell to the next very rapidly. This is
most likely accomplished by a mechanism
analogous to a(n):
A. Chemical synapse
B. Electrical synapse
B. Electrical synapse
A mutation in the gene for synaptotagmin has been
found in a few individuals with movement and
cognitive disorders. Research into the impact of
these mutations on neuronal function revealed that
some variants caused which of the following effects
when the presynaptic neuron was stimulated?
A. Closing of gap junctions on the presynaptic
B. Slowed rate of exocytosis by the presynaptic neuron membrane
C. Hyperpolarization of the postsynaptic membrane
D. Impaired calcium influx in the presynaptic neuron
E. Increased endocytosis by the presynaptic neuron
B. Slowed rate of exocytosis by the presynaptic neuron membrane
Major types of receptors (2)
Ionotropic
Metabotropic
Ionotropic
Receptors that contain an ion channel
Metabotropic
Receptors that are coupled to G proteins or other intracellular signaling pathways
G protein coupled receptors Example (1)(1)
Muscarinic receptors
On brain neurons, heart, smooth muscle, glands
Ligand-gated ion channels example (1)(1)
Nicotinic receptors
On brain neurons, Also on skeletal muscle and post synaptic neurons of the autonomic nervous system
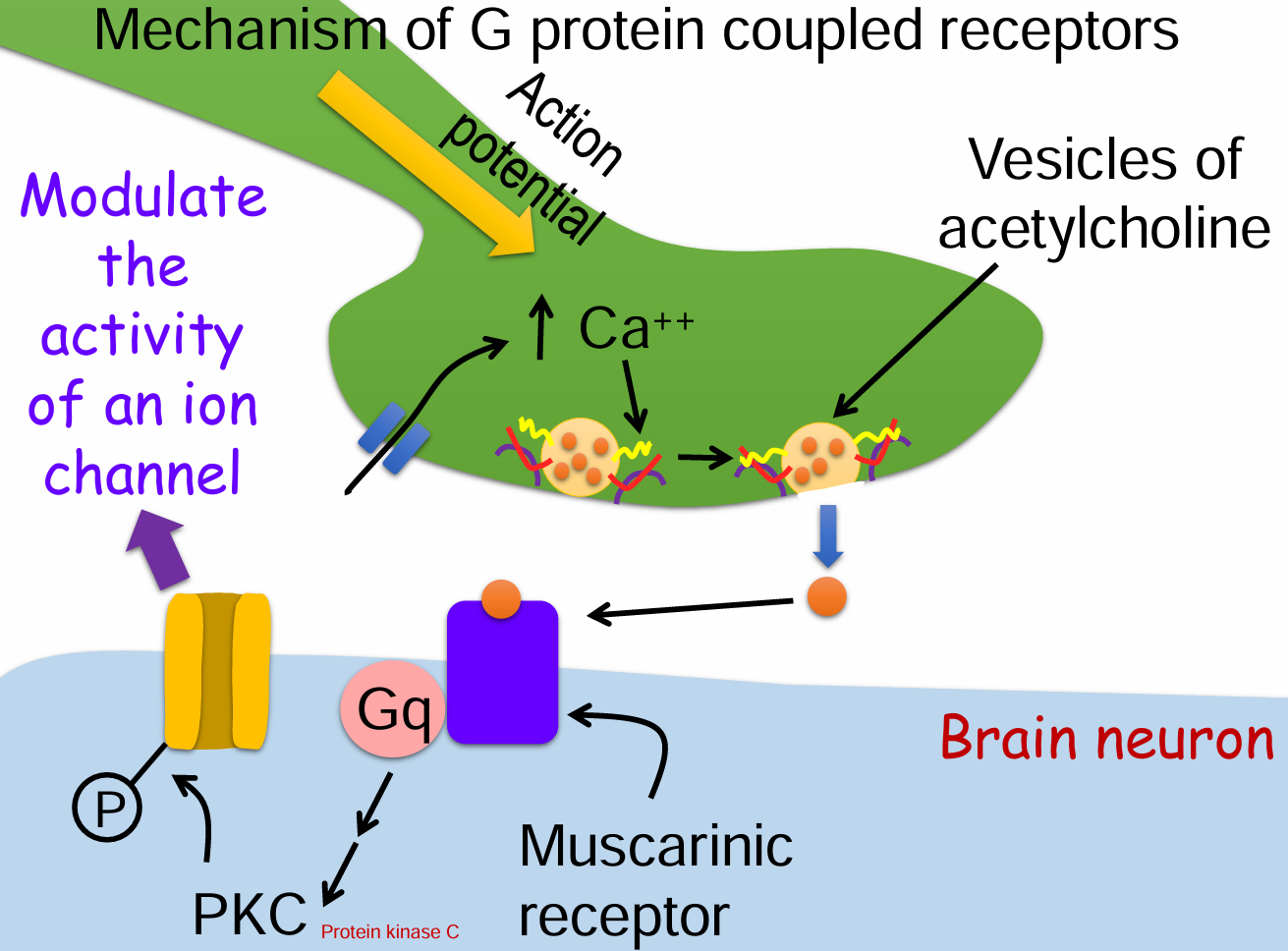
Mechanism of G protein coupled receptors (7)
Action potential arrives at the presynaptic terminal.
Voltage-gated Ca²⁺ channels open → Ca²⁺ influx.
Vesicles release acetylcholine (ACh) into the synaptic cleft.
ACh binds to muscarinic GPCR on the postsynaptic membrane.
Gq protein is activated → triggers a signaling cascade.
Protein kinase C (PKC) is activated.
PKC phosphorylates and modulates an ion channel’s activity.
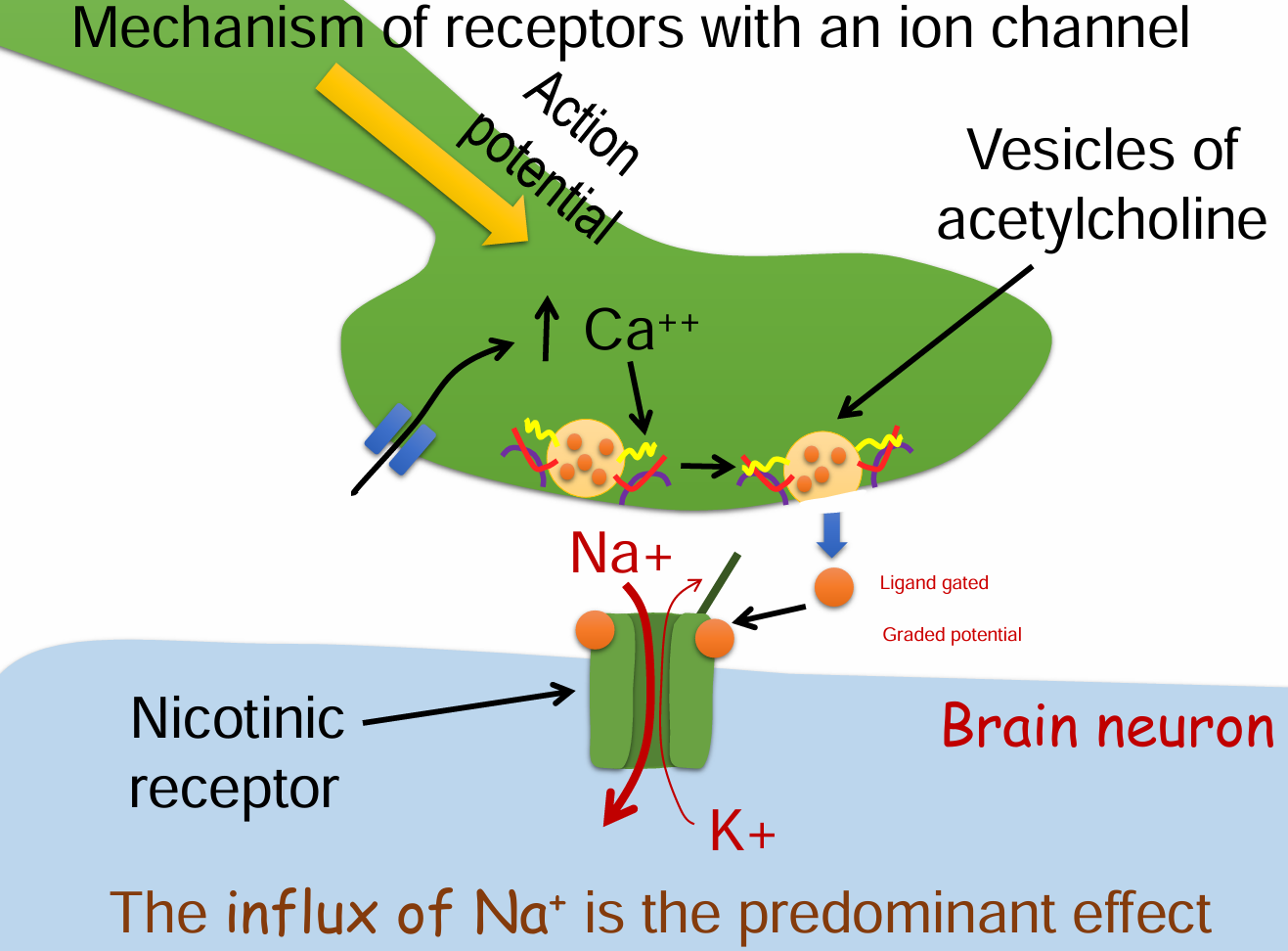
Mechanism of receptors with an ion channel (6)
Action potential arrives at the presynaptic terminal.
Voltage-gated Ca²⁺ channels open → Ca²⁺ influx.
Vesicles release acetylcholine (ACh) into the synaptic cleft.
ACh binds to nicotinic receptors (ligand-gated ion channels) on the postsynaptic membrane.
The channel opens → Na⁺ flows in (predominant), K⁺ flows out.
The net influx of Na⁺ depolarizes the postsynaptic neuron → graded potential.
The activation of the nicotinic acetylcholine receptor cause
Membrane depolarization
What is the main depolarizing current on the postsynaptic membrane of a neuron? What channel? What potential?
Na+
Ligand gated channel
Graded potential
2 types of synaptic potentials on the post synaptic membrane
Excitatory postsynaptic potential (EPSP)
Inhibitory postsynaptic potential (IPSP)
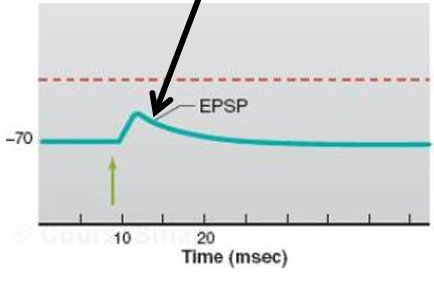
Is an EPSP caused by a localized depolarization or hyperpolarization?
depolarization
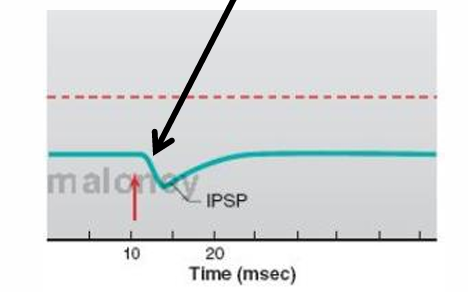
Is an IPSP caused by a localized depolarization or hyperpolarization?
hyperpolarization
Any neurotransmitter that activates channels on the postsynaptic membrane that let both Na+ and K+ through will produce an
EPSP
Which of the following can cause an IPSP? (2)
Opening a Cl- channel
In cells with a chloride pump that actively transports Cl- out of the cell
Opening a K+ channel
What does the equilibrium potential for an ion need to be in order to cause an IPSP
More negative than the resting membrane potential
The ionotropic receptor for the neurotransmitter GABA contains a Cl- channel. Is GABA an excitatory or inhibitory neurotransmitter?
inhibitory
The NMDA receptor for the neurotransmitter glutamate contains a Ca++ channel. Is glutamate an excitatory or inhibitory neurotransmitter?
excitatory
Brainstorm 3 ways of getting the neurotransmitter out of the synaptic cleft?
Reuptake into the nerve terminal
Enzymatic degradation
Diffuse out
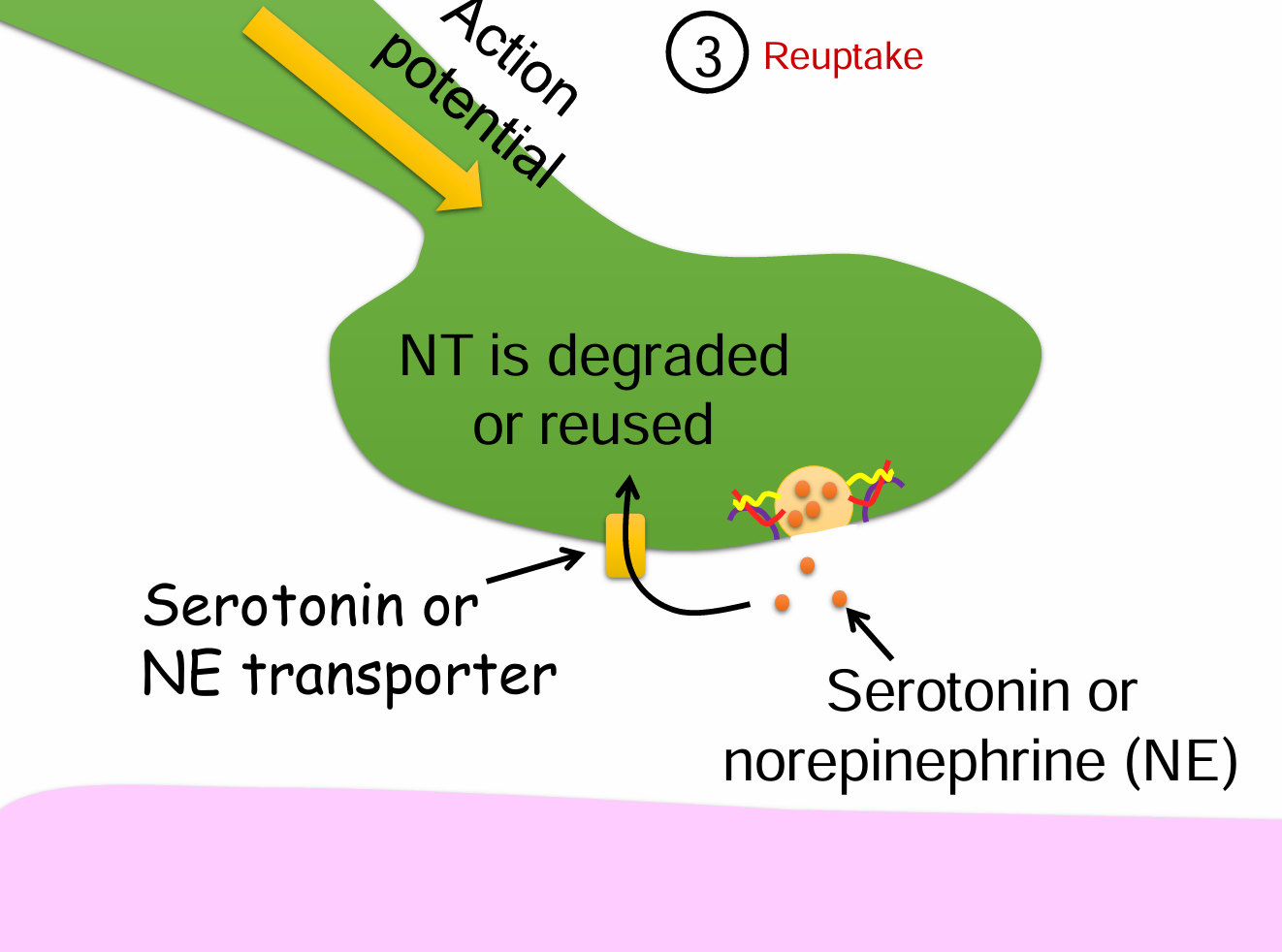
Reuptake into the nerve terminal steps (4)
Action potential reaches the presynaptic terminal.
Serotonin or norepinephrine (NE) is released into the synaptic cleft.
A transporter protein (e.g., serotonin or NE transporter) reabsorbs the neurotransmitter.
Neurotransmitter is either degraded or reused inside the presynaptic neuron.
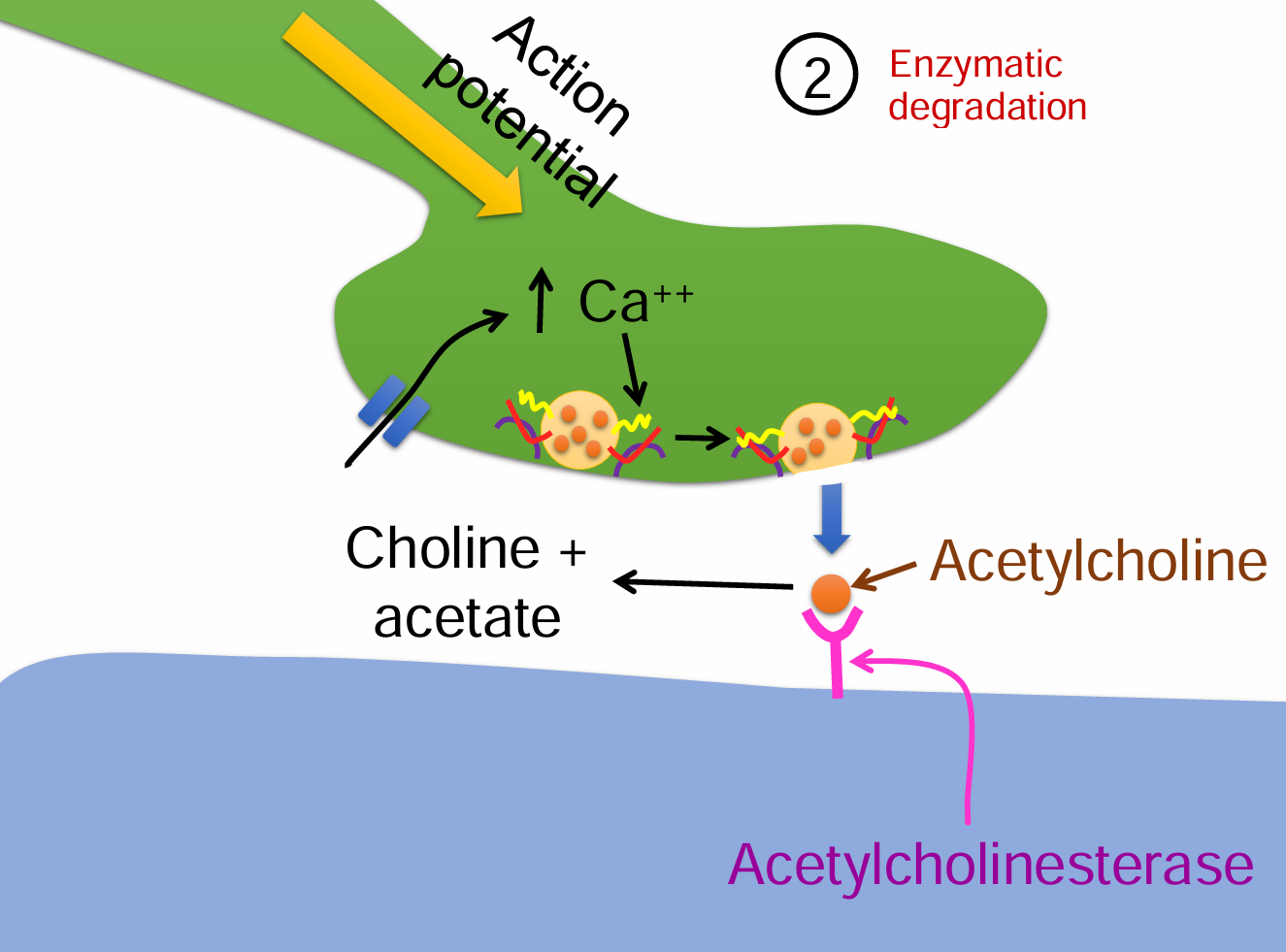
Enzymatic degradation steps (5)
Action potential triggers Ca²⁺ influx into the presynaptic terminal.
Vesicles release acetylcholine (ACh) into the synaptic cleft.
ACh binds to receptors but is then rapidly broken down by acetylcholinesterase.
ACh is split into choline + acetate.
Choline is taken back up and reused for new ACh synthesis.
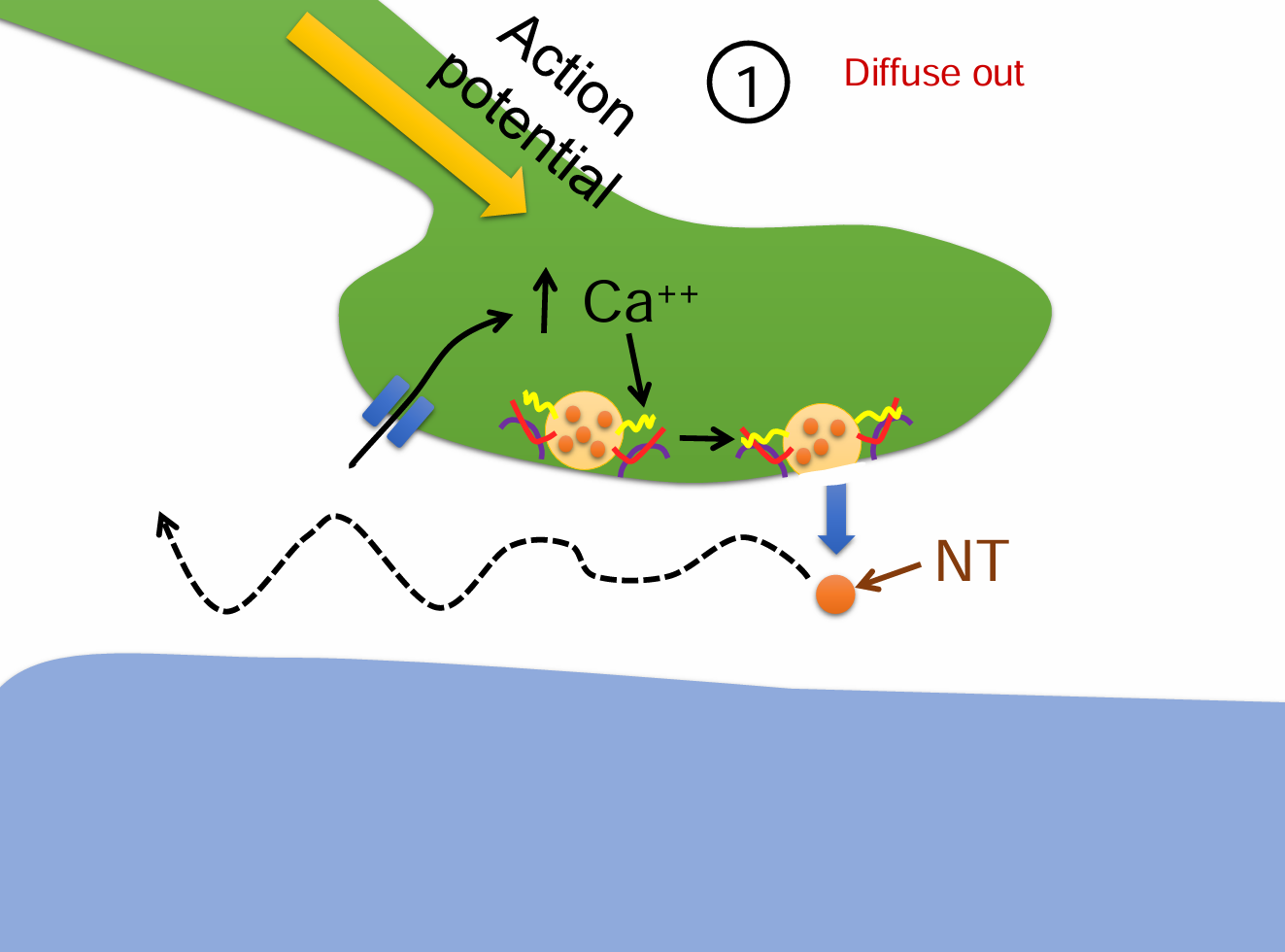
Diffuse out steps (4)
An action potential triggers Ca²⁺ influx into the presynaptic terminal.
Neurotransmitters (NTs) are released into the synaptic cleft.
Instead of binding or being degraded, some NT molecules passively diffuse away from the synaptic cleft.
This process reduces neurotransmitter concentration and terminates signaling.
Divergence
Gives input to many (maybe hundreds) of other neurons
convergence
Receives input from many (maybe hundreds) of other neurons
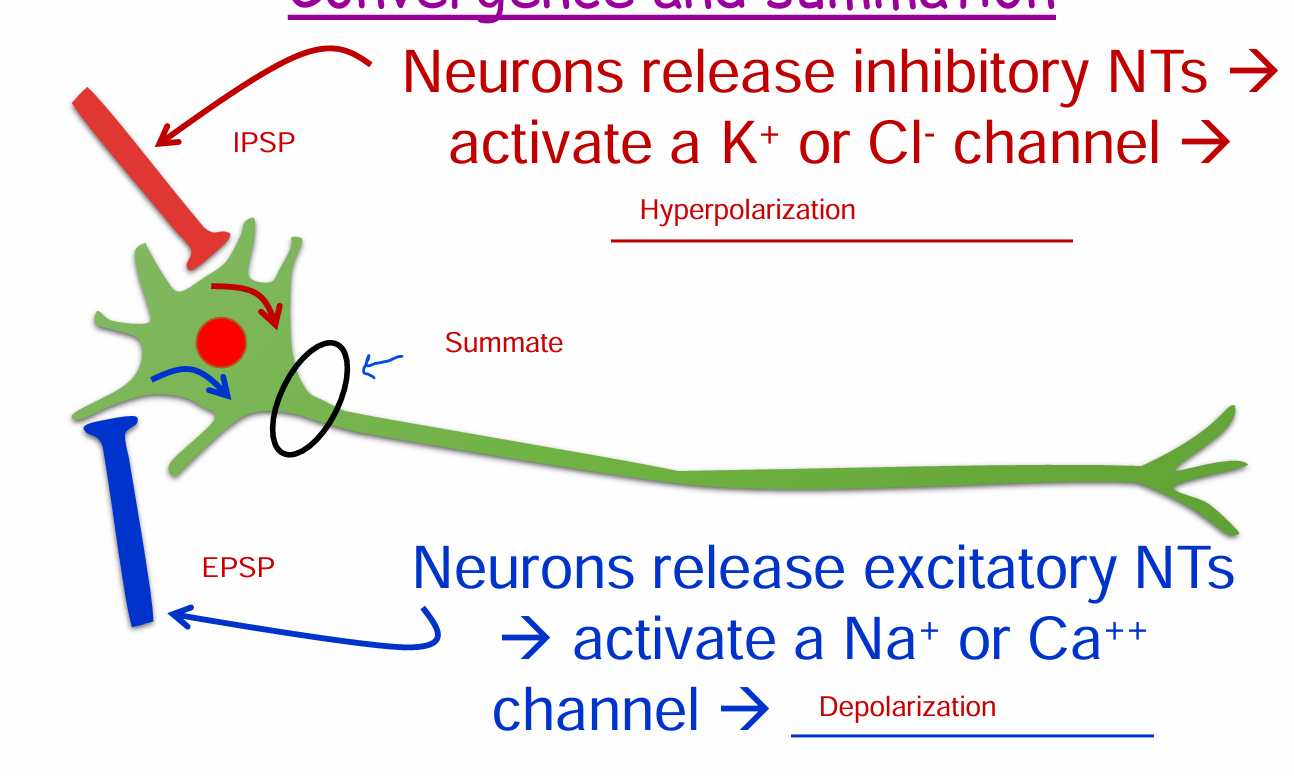
Convergence and summation
IPSP: Neurons release inhibitory NTs → activate a K+ or Cl- channel → Hyperpolarization
EPSP: Neurons release excitatory NTs → activate a Na+ or Ca++ channel → Depolarization
When is an action potential generated?
If the axon hillock reaches threshold
Modes of summation (2)
Temporal summation
Spatial summation
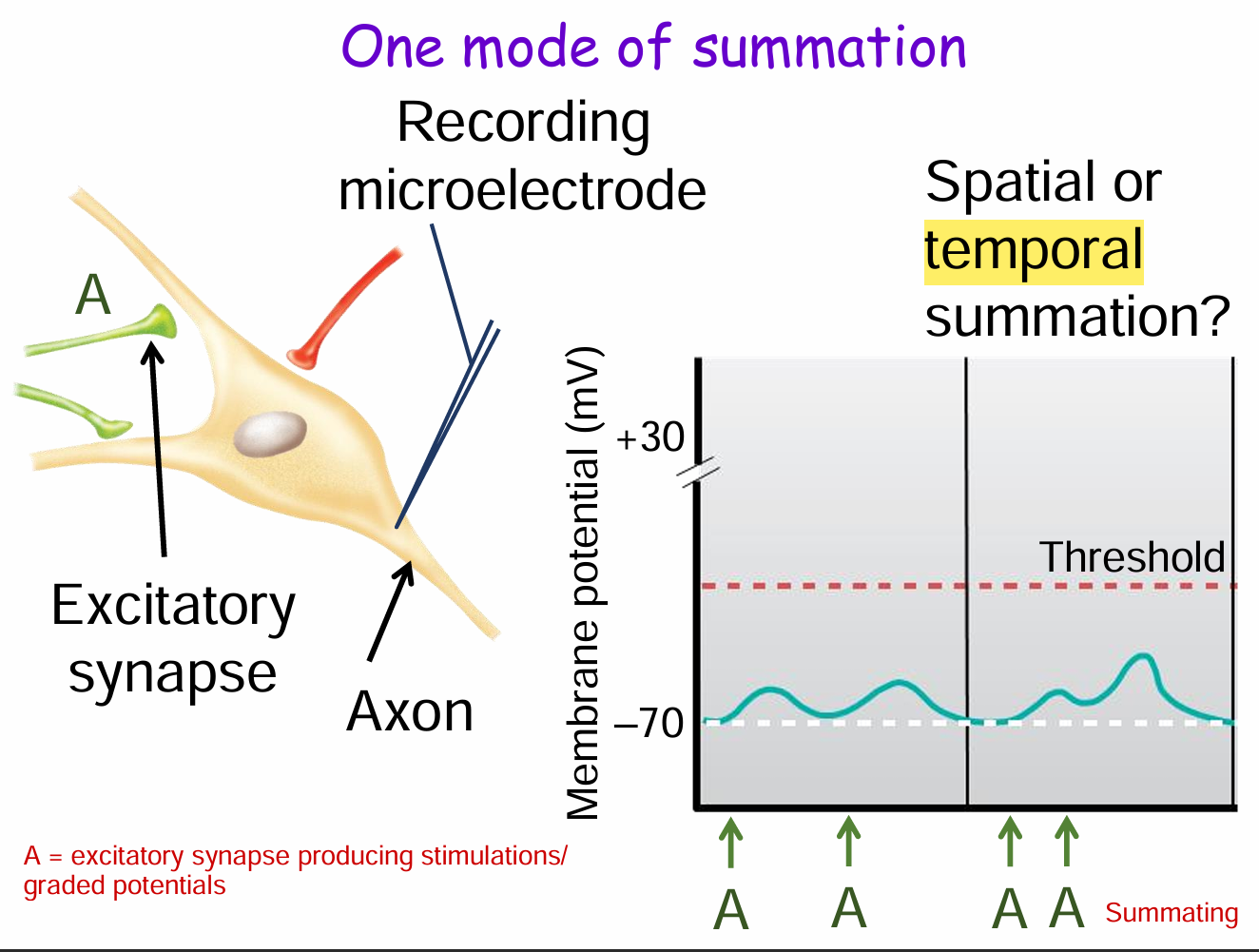
What is temporal summation and how is it shown in this diagram?
Temporal summation is when a single presynaptic neuron (e.g., synapse A) fires repeatedly over time, causing graded potentials to build up at the postsynaptic neuron.
In the diagram, the membrane potential increases stepwise with each stimulus from A until it reaches threshold, potentially triggering an action potential.
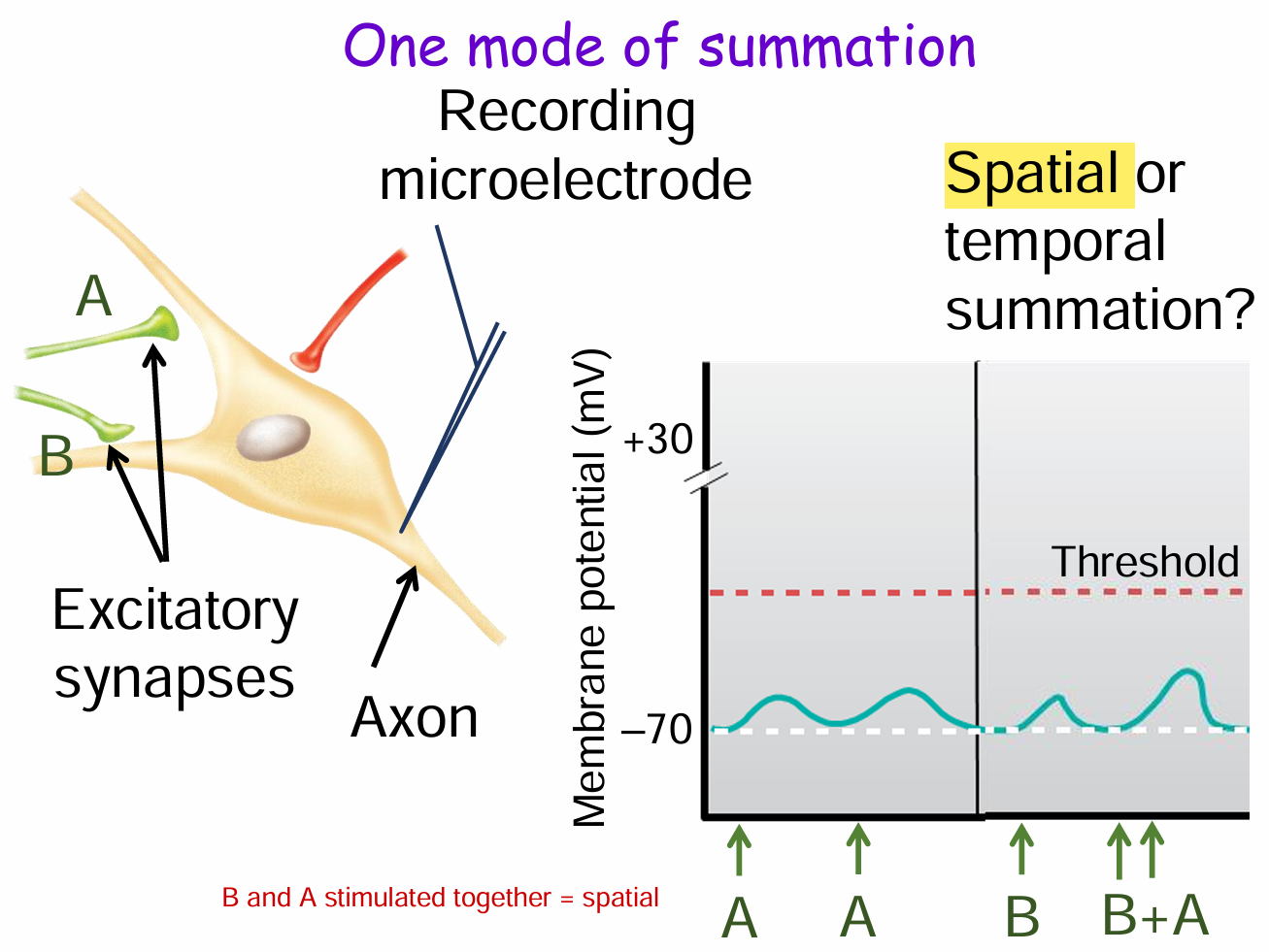
What is spatial summation and how is it shown in this diagram?
Spatial summation occurs when multiple presynaptic neurons (e.g., synapses A and B) fire at the same time, and their graded potentials combine at the postsynaptic neuron.
In the diagram, stimulation from A and B together produces a larger depolarization than either alone, pushing the membrane potential closer to threshold.
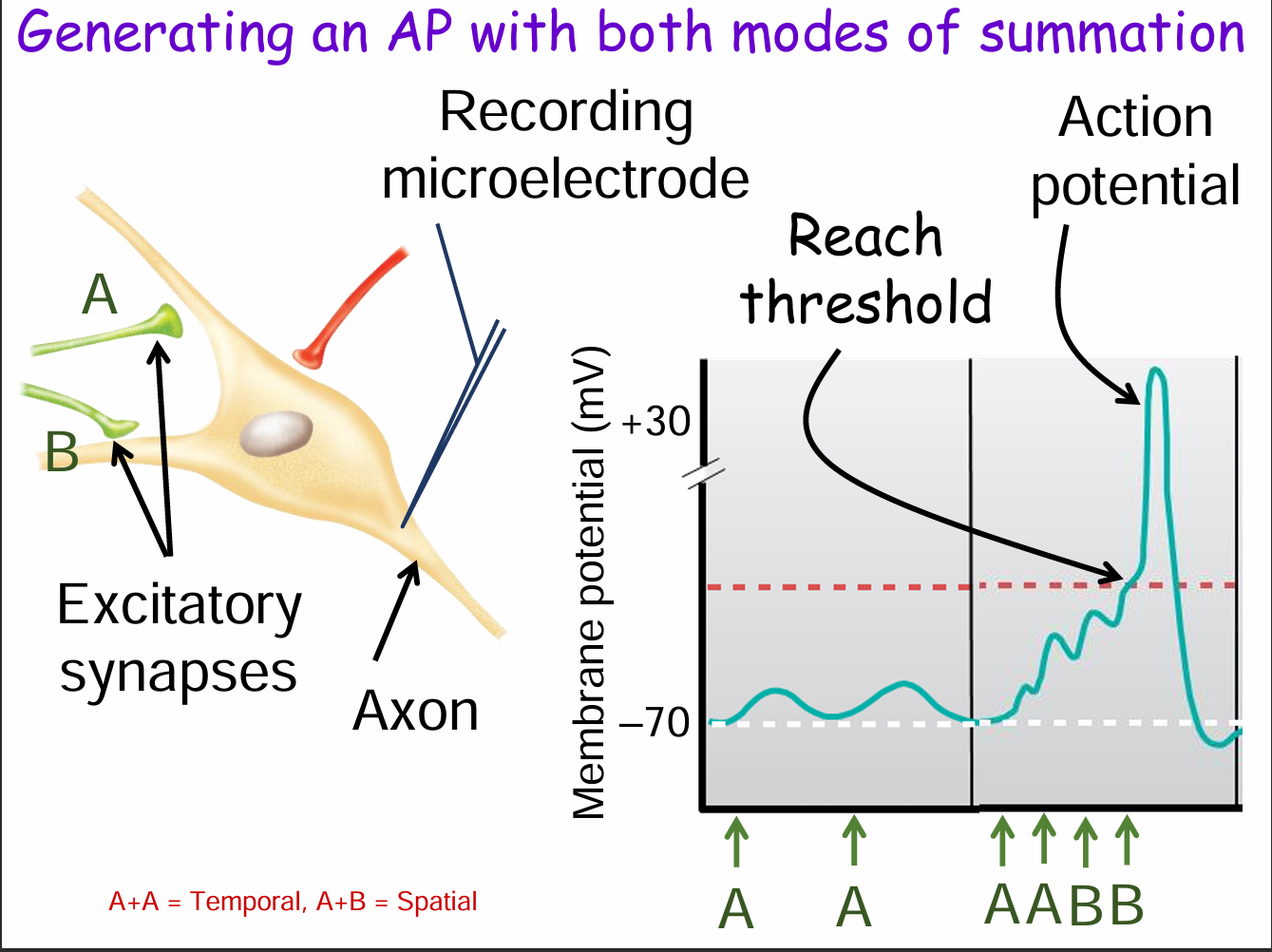
How can both spatial and temporal summation together trigger an action potential? (3)
Temporal summation: Repeated input from the same synapse (e.g., A + A).
Spatial summation: Combined input from multiple synapses (e.g., A + B).
Together, these raise the membrane potential enough to reach threshold, triggering an action potential.
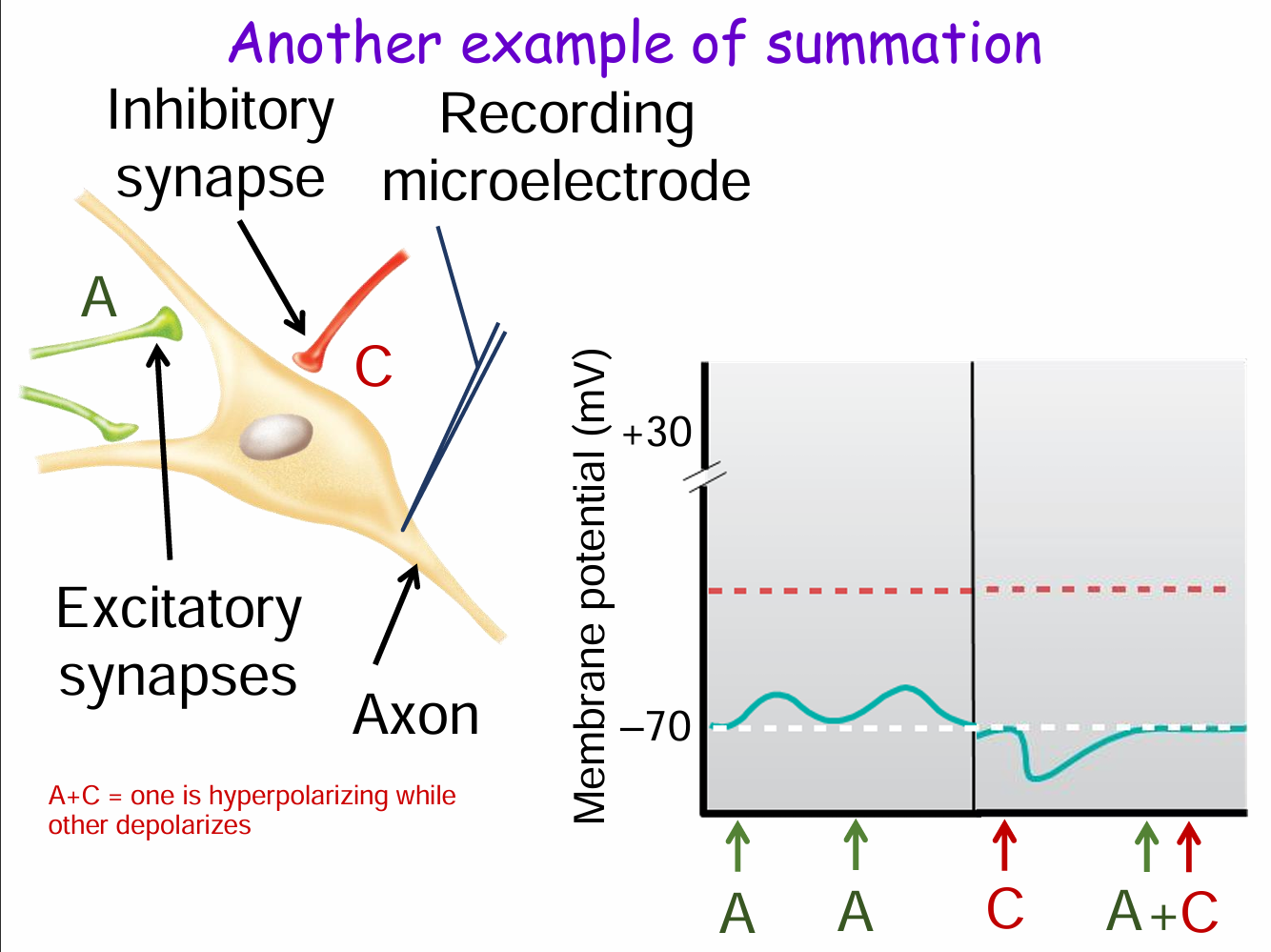
What happens when excitatory and inhibitory synapses are activated together (e.g., A + C)? (3)
Synapse A is excitatory (depolarizes membrane).
Synapse C is inhibitory (hyperpolarizes membrane).
When both fire together (A + C), their effects cancel out, preventing the membrane potential from reaching threshold and inhibiting an action potential.
Some things that can modify the synaptic strength (3)
Modify the release of the NT presynaptic mechanism
Modify receptor activity postsynaptic mechanism
Diseases or drugs!!!!!
Presynaptic mechanisms that change synaptic strength (4)
High frequency stimulation
Autoreceptors
Chemical messengers released from other cells
Axo-axonic synapses
High frequency stimulation
Not enough time to pump out the Ca++ → Next action potential → greater increase in intracellular Ca++ → greater NT release → greater EPSP or IPSP
Autoreceptor
Typically decrease NT release
Axo-axonic synapse
Can increase or decrease NT Release
Messenger from a nearby neuron
Can increase or decrease NT release
All presynaptic mechanisms can change the amount of NT released from neuron B, and alter the response of neuron C by
Changing the amount of Ca++ That enters the cell
Postsynaptic mechanisms that change synaptic strength
Desensitization of receptors
Up regulation of receptors
Down regulation of receptors
What causes desensitization of receptors?
Ligand binding to a receptor → Causes a change in the receptor e.g. it is phosphorylated → Decrease the sensitivity of the receptor to subsequent stimulation
Concrete example: Drug abusers need more drug to get the same effect. Tolerance.
Up regulation (2)
Caused by chronic exposure to a chemical messenger or drug that blocks receptor activation
Body reacts with more receptors place on the membrane
Down regulation (2)
Caused by chronic exposure to a chemical messenger or drug that activates a receptor
Body reacts with receptors internalized/ degraded → less receptors on the membrane
While the pathogenesis of Alzheimer's disease is multifactorial and not completely elucidated, glutamate, the main excitatory amino acid in the brain which is involved in long-term potentiation (a major process involved in learning and memory), may contribute to this disease by overstimulating the NMDA receptor (one of 2 main types of glutamate receptors) leading to excitotoxicity and neuronal cell death. Memantine is a drug that is useful in the treatment of Alzheimer’s disease because it:
A. Blocks NMDA receptor and reduces Ca++ influx
B. Blocks NMDA receptor and increases Ca++ influx
C. Activates NMDA receptor and reduces Cl- influx
D. Activates NMDA receptor and increases Cl- influx
A. Blocks NMDA receptor and reduces Ca++ influx
Excitatory = depolarize = Ca++
Reduce = less depolarization/ excitation
Acts as an antagonist
Acetylcholine (ACh) is an excitatory neurotransmitter in the brain. It is released from cholinergic neurons. One thing it is involved in, is learning and memory. Alzheimer’s disease is associated with the loss of cholinergic neurons in parts of the brain. Donepezil (Aricept) is useful in treating Alzheimer’s because it most likely:
A. Inhibits acetylcholinesterase
B. Inhibits Ca++ influx on presynaptic
cholinergic neurons
C. Desensitizes muscarinic receptors
D. Down regulates nicotinic receptors
E. Stimulates the reuptake of ACh
A. Inhibits acetylcholinesterase
Want to give them a drug that helps with memory. Helps prevent breakdown of ACh
Tetanus toxin causes a rigid or spastic paralysis by preventing the release of the inhibitory neurotransmitter GABA from a presynaptic terminal because the toxin most likely:
A. Facilitates the binding of Ca++ to synaptotagmins
B. Up regulates voltage gated Ca++ channels on the axon terminal
C. Degrades a SNARE protein
D. Desensitizes the GABA receptor
E. Blocks the Cl- channel associated with the GABA receptor
C. Degrades a SNARE protein
Presynaptic effect, less exocytosis
Serotonin is a neurotransmitter in the brain. One thing it is involved with is emotional states, such as mood and anxiety. Fluoxetine (Prozac) is a selective serotonin reuptake inhibitor (SSRI) that is used for depression. What effect will it have (either directly or indirectly) on the activity of the serotonin receptor in the brain?
Increase its activity
Both barbiturates and benzodiazepines (such as diazepam (Valium)) can cause sedation by facilitating or increasing the effect of the main inhibitory neurotransmitter, GABA, in the brain. What is most likely happening with this?
A. Valium causes an increase in GABA
induce Na+ influx
B. Valium causes a decrease in GABA
induce Na+ influx
C. Valium causes an increase in GABA
induce Cl- influx
D. Valium causes a decrease in GABA
induce Cl- influx
C. Valium causes an increase in GABA induce Cl- influx
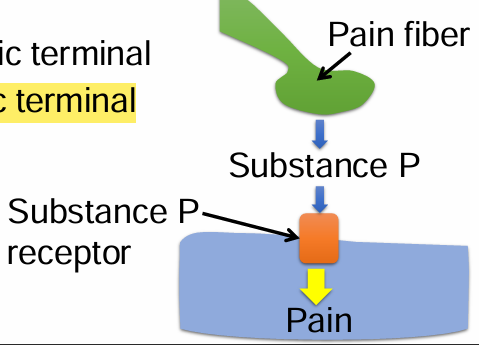
Substance P is a neuropeptide involved in pain sensation. Beta-endorphins bind to and activate opioid receptors. Activation of opioid receptors prevents the release of substance P from pain fibers, and hence inhibit pain. Where is the opioid receptor located?
A. Postsynaptic terminal
B. Presynaptic terminal
B. Presynaptic terminal
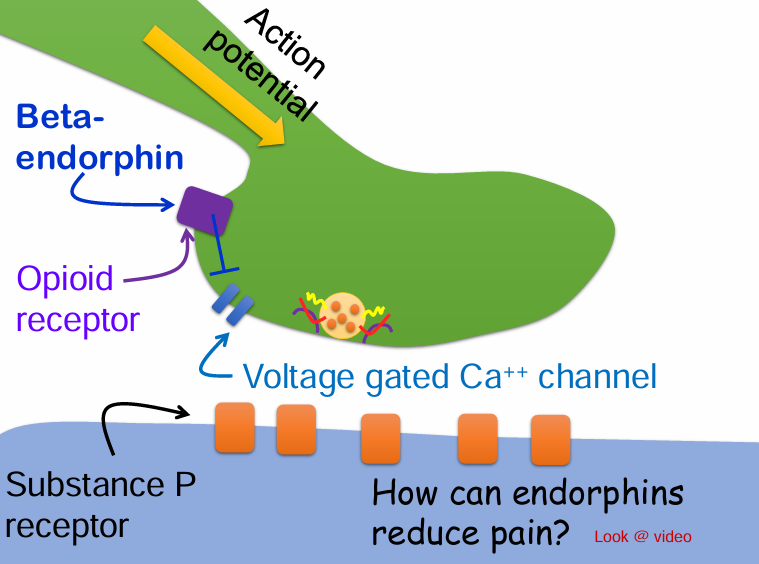
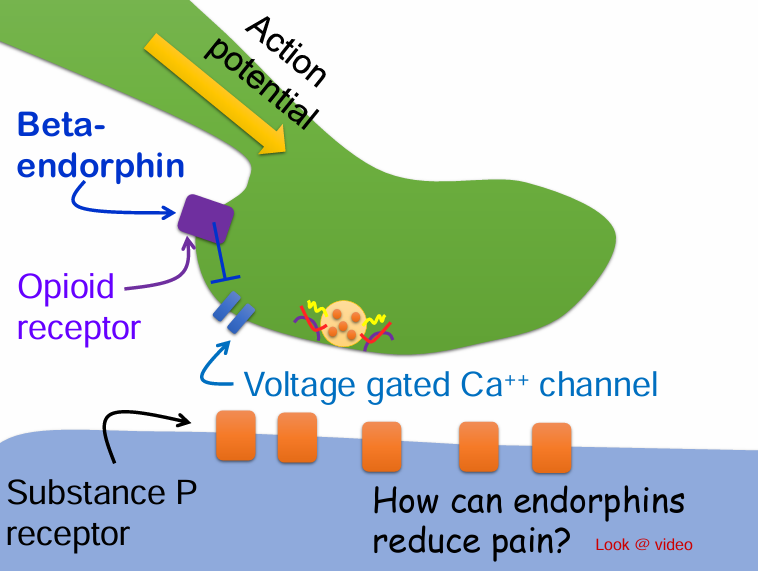
Would beta-endorphin be considered a neurotransmitter or neuromodulator?
A. Neurotransmitter
B. Neuromodulator
B. Neuromodulator
Often modify the possynaptic cells response to a NT
In a chemical synapse, is the post synaptic cell always a neuron?
No, it can also be a muscle cell or gland → Neuroeffector junction