Extraction of Aluminium
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What is an aluminum ore called?
bauxite
What happens when we purify bauxite?
becomes a white powder - aluminium oxide from which aluminium can be extracted.
The extraction is done by electrolysis, but first the aluminium oxide must be 1.______ so that electricity can pass through it.
Why is obtaining aluminium by elctrolysis very expensive/ why is aluminium expensive even though it is the most abundant metal on Earth?
melted
it has a very high melting point (over 2,000 degrees Celsius) so it would be very expensive to melt it as it requires a lot of energy.
what is molten cryolite and why is it used.
It is an aluminium compound with a lower melting point than aluminium oxide.
The use of molten cryolite as a solvent reduces some of the energy costs involved in extracting aluminium by allowing the ions in aluminium oxide to move freely at a lower temperature.
Here are one of the forms of electrolysis.
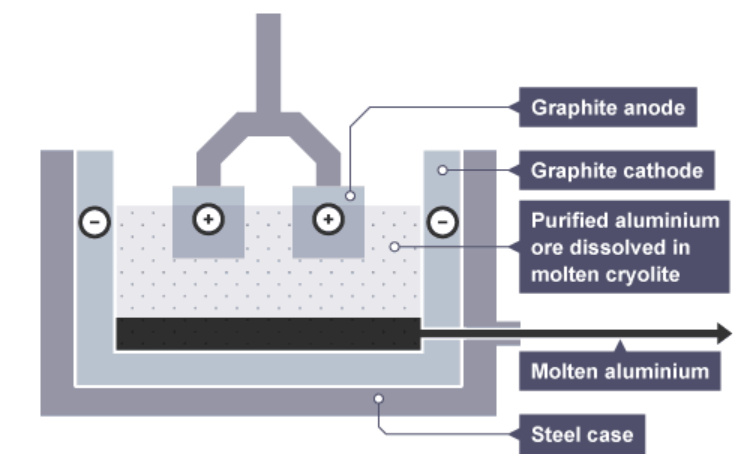
Aluminium ions receive electrons at the negative electrode and are reduced to aluminium atoms:
What is the half equation of the aluminium?
what type of reaction is this?
Al3+ + 3e– → Al (reduction – gain electrons)
Oxide ions lose electrons at the positive electrodes and are oxidised to oxygen gas:
What is the half equation for this?
What type of reaction?
2O2– → O2 + 4e– (oxidation – lose electrons)
Why does oxygen being given off increase the cost f the process?
This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. As a result, the positive electrodes have to be replaced frequently. This adds to the cost of the process.
There are a number of important factors to consider when choosing the site of an aluminium extraction plant. It should be:
At least 3/5
in close proximity to a power station, in order to provide the large supply of electricity needed for the electrolysis
near the coast to allow for the import of raw materials
near roads and railway lines to allow for the product to be taken to where it is needed
near a town or city, so that workers have somewhere to live close by
away from built-up areas, so that the noise and pollution of the site do not affect the local population