Cell Bio (pt1) Cell Division, Migration, and Apoptosis Handout
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
3 different cell behaviors controlled by signaling
- grow and divide
- migrate
- die
-> signaling works in a combinatorial fashion in inter-connected networks
5 main phases of mitosis
1. prophase
2. metaphase
3. anaphase
4. telophase
5. cytokinesis
human body experiences how many cell divisions in a lifetime?
10 quadrillion
how does a cell trigger events to start mitosis?
mitotic protein kinases
- a positive feedback loop helps keep these kinases active during mitosis
mitotic protein kinases
- multiple aurora kinases (A, B, C)
- multiple polo-like kinases (1-4)
-> these are dysregulated in different cancers
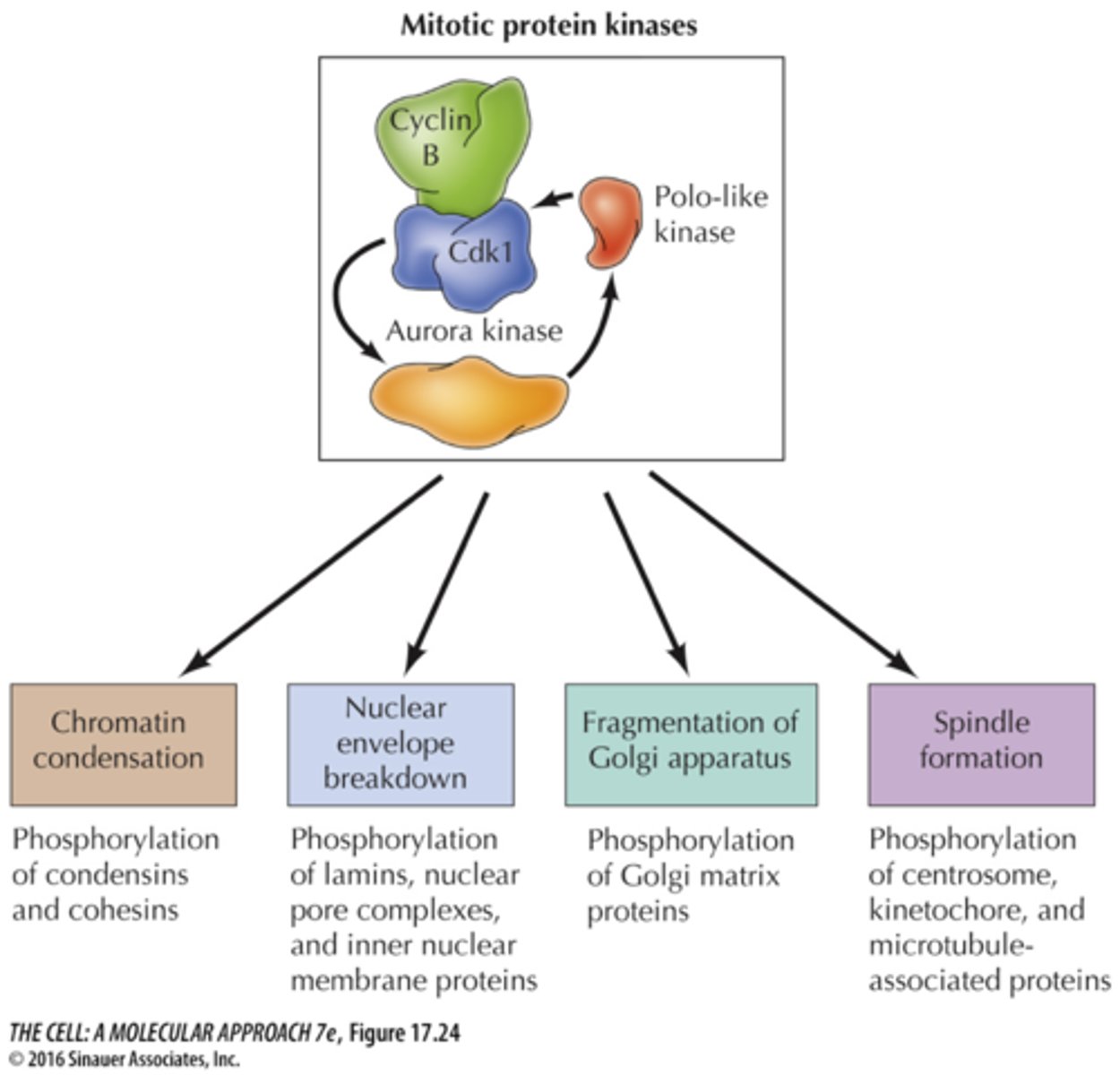
mitotic protein kinases lead to which actions?
- chromatin condensation
- nuclear envelope breakdown
- fragmentation of golgi apparatus
- spindle formation
chromatin condensation
phosphorylation of condensins and cohesins
- caused by mitotic protein kinases
nuclear envelope breakdown
phosphorylation of lamins, nuclear pore complexes, and inner nuclear membrane proteins
- caused by mitotic protein kinases
fragmentation of golgi apparatus
Phosphorylation of Golgi matrix proteins
- caused by mitotic protein kinases
spindle formation
Phosphorylation of centrosome, kinetochore, and microtubule-associated proteins
- caused by mitotic protein kinases
prophase
first step of mitosis
1. chromosomal material condenses to form compact mitotic chromosomes
- chromosomes are seen to be composed of two chromatids attached together at the centromere
2. cytoskeleton is disassembled, mitotic spindle is assembled
3. golgi complex and ER fragment
- nuclear envelope disperses

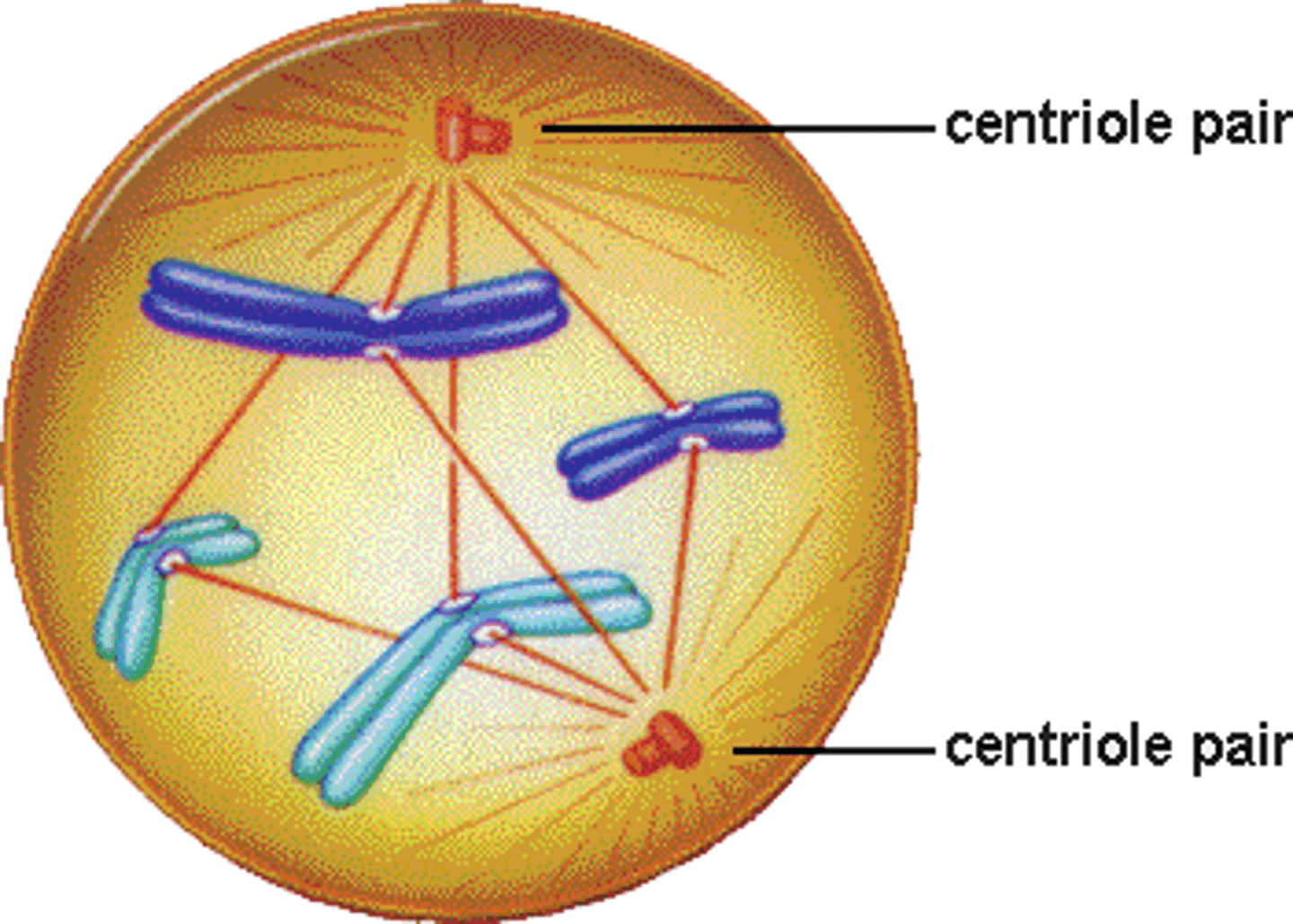
prometaphase
after prophase
1. chromosomal microtubules attach to kinetochores of chromosomes
2. chromosomes are moved to spindle equator
- condensed chromosomes are scattered in early prometaphase
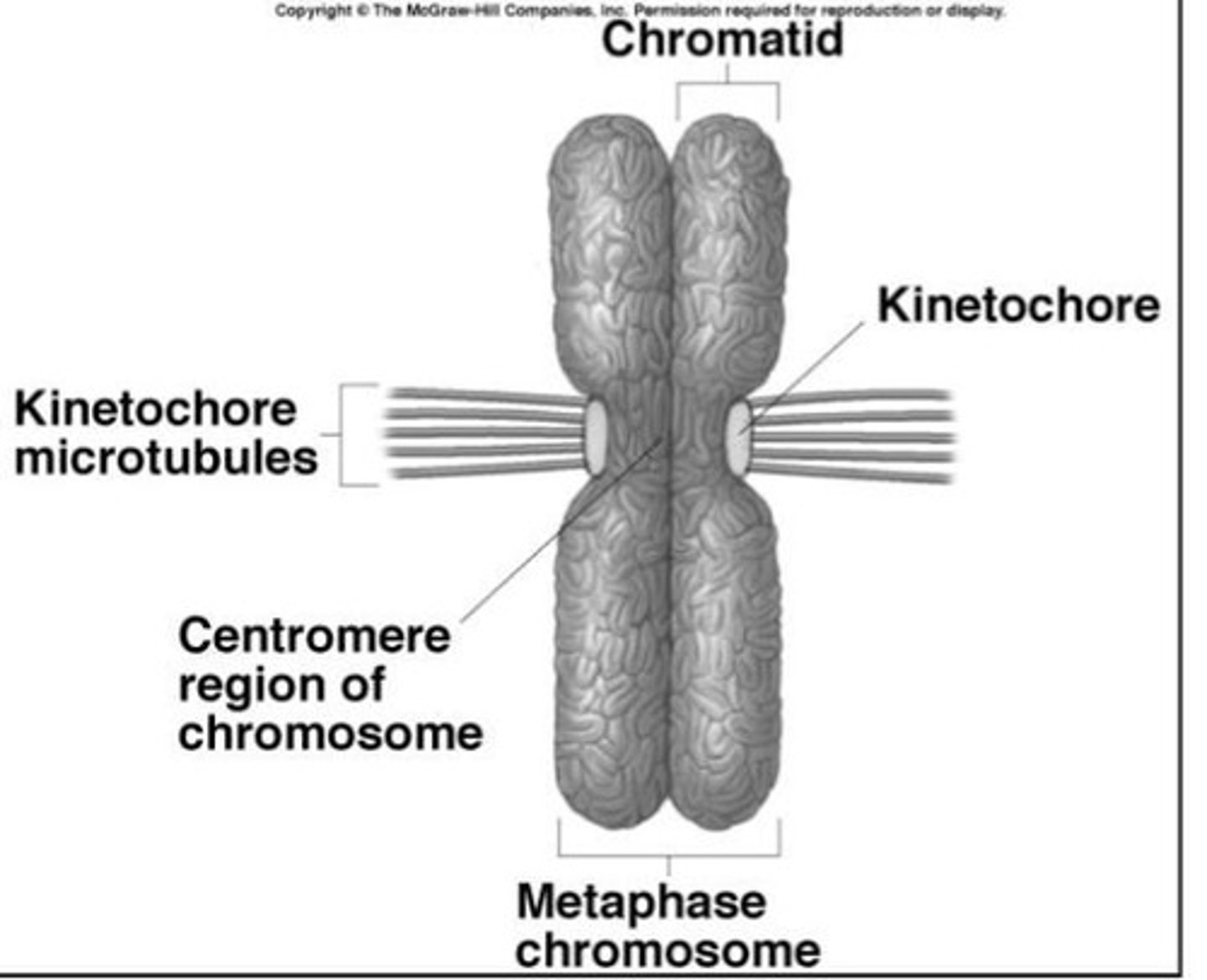
condensed mitotic chromosome
- 2 chromatids
- centromere
cohesins
hold sister chromatids together
- form rings around the chromatids from S-phase to anaphase
interphase
S/G2-phase
condensins
initiate chromosome compaction
- form rings around the supercoiled DNA loops
just as chromatids must be duplicated, centrosomes must be duplicated so there are two- why?
helps insure mitosis will be normal
centrosome duplication and separation associated with the cell cycle
- duplication starts at late G1 phase and is finished by the end of S-phase
- by late G2 phase the centrosomes "mature"
- during mitosis, the microtubule motor kinesin-5 [(+) end] is involved pushing the centrosomes apart. also kinesin-12
duplication occurs when?
duplication starts at late G1 phase and is finished by the end of S-phase
duplication starts at late G1 phase and is finished by the end of S-phase
controlled by phosphorylation of centrosome proteins by Cdk2 and others
by late G2 phase the centrosomes "mature"
acquire PCM and become phosphorylated
- helps recruit more γ-tubulin to support microtubule nucleation during mitosis (MPF, and in particular PLK4 and AuroraA)
-> the centrosomes begin to separate from each other in late G2 phase
the centrosomes begin to separate from each other in late G2 phase. how?
- Myosin II and cytoplasmic dynein-1 are involved
- maybe force from microtubule polymerization also
what happens if we block centrosome separation during mitosis?
kinesin-5 is required for centrosome separation and spindle bipolarity
- cannot divide
what might happen if a cell that has too many centrosomes tries to divide?
arrest during S-phase
building the mitotic machine: the bipolar mitotic spindle
prometaphase
- centrosomes continue to separate and move toward opposite ends of the cell
- phosphorylation of PCM proteins by mitotic kinases is thought to play a key role in stimulating nucleation of spindle microtubules
- dissolution of the nuclear envelope is complete
- microtubular growth and molecular motors form the mitotic spindle
as the microtubules of the spindle penetrate into the chromatin mass, what happens?
the free (+) ends of the microtubules undergo dynamic instability, as if they were "searching" for a chromosome
- it is not certain whether searching is entirely random. evidence suggests that microtubules may grow toward a site containing certain chromatin
how do the chromosomes become aligned to achieve metaphase?
congression
congression
CHROMOSOMES ARE MOVED TO CENTER OF MITOTIC SPINDLE
kinetochores assemble at each centromere and attach each chromatid to microtubule bundles- the metaphase plate is formed
-> the dynamic microtubules that contact a kinetochore are "captured" and stabilized- "kinetochore fibers"
the kinetochore
>100 proteins
- 20-30 mts
- started assembling in prophase
- mitotic kinases
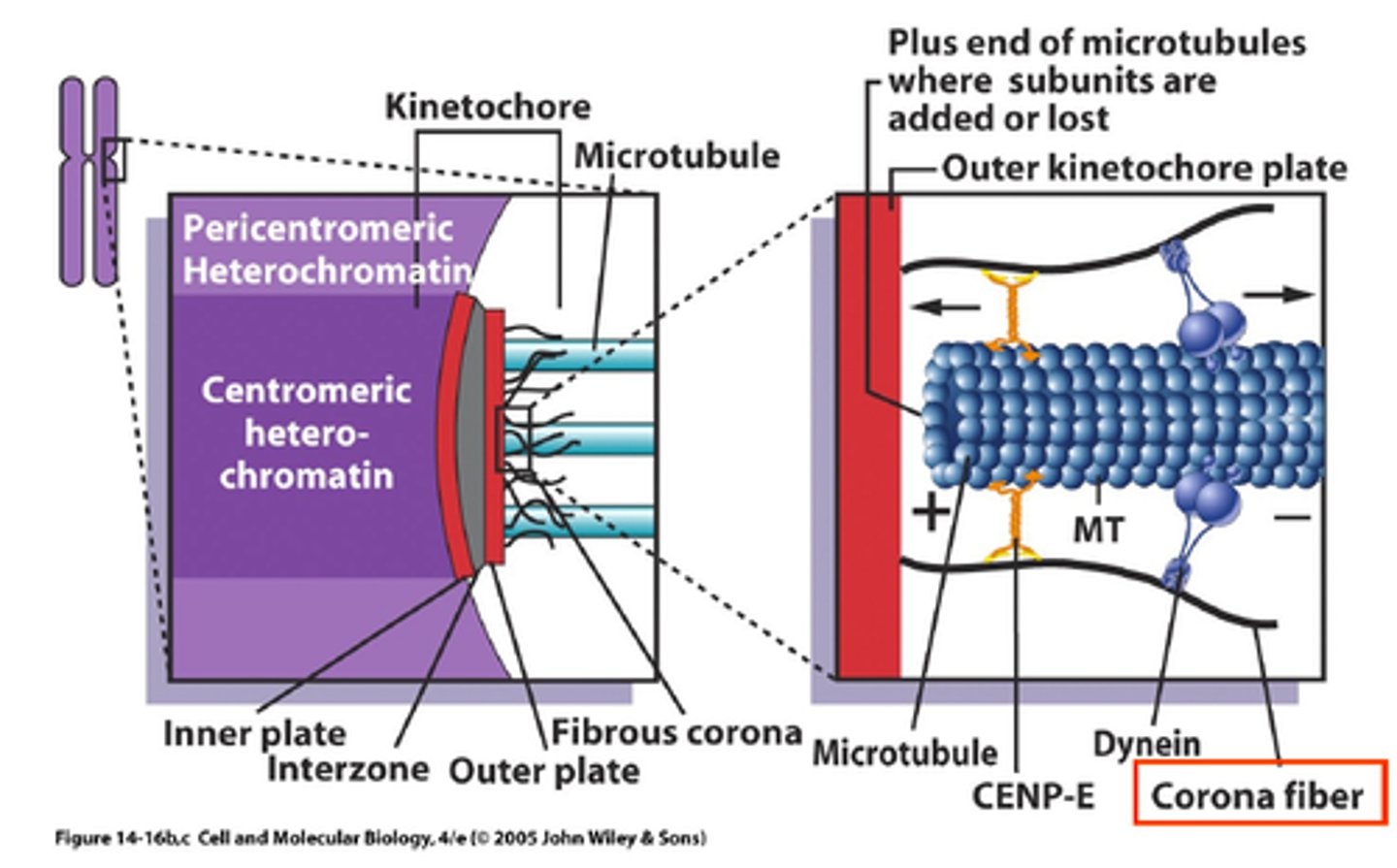
outer plate of kinetochore
- MT binding
- MT motor activity
- signal transduction
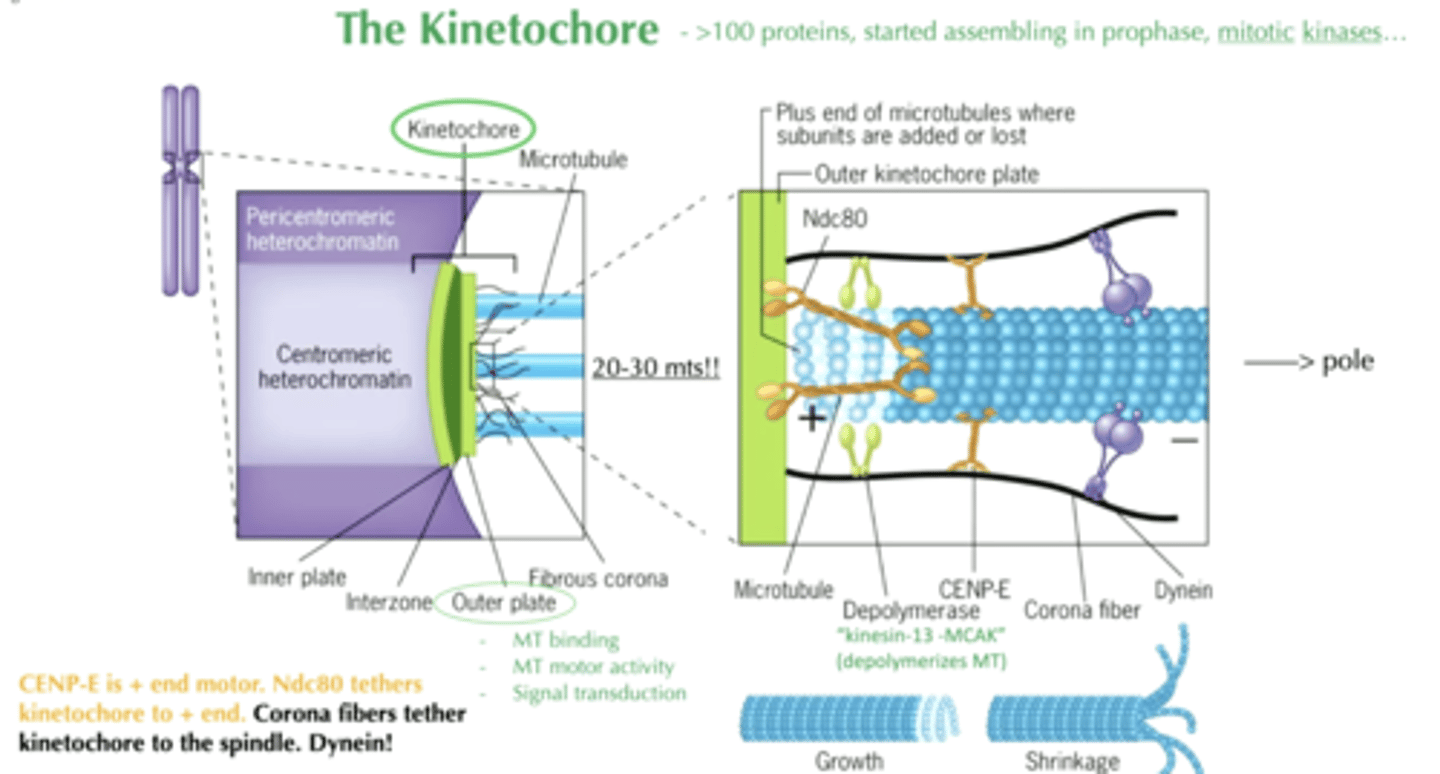
depolymerase on kinetochore
"kinesin-13 MCAK"
- depolymerized MT
CENP-E
+ end motor
Ndc80
tethers kinetochore to + end
corona fibers
tether kinetochore to the spindle
+ end of microtubules
where subunits are added or lost
during prometaphase, what must happen?
chromosomes and microtubules must attach properly
during prometaphase, chromosomes and microtubules must attach properly
1. kinetochore contacts the sidewall of MT and slides along MT using its motors
2. mono-oriented chromosome (no tension)
3. bi-oriented chromosome (under tension)
3a. syntelic attachment (no tension)
4. equal tension applied by microtubules
chromosome and microtubule attachment in prometaphase: step 2. mono-oriented chromosome (no tension)
chromosome attached to the + end of MT bundle from 1 spindle pole
chromosome and microtubule attachment in prometaphase: step 3. bi-oriented chromosome (under tension)
mitotic spindle assembly completes and chromosomes are moved into position at the center of the cell
chromosome and microtubule attachment in prometaphase: step 3a. syntelic attachment (no tension)
2 MT bundles attach from the same spindle pole
chromosome and microtubule attachment in prometaphase: step 4. equal tension applied by microtubules
oriented at the center of the cell
- "congression"
initially, chromosomes are attached to what?
kMTs that differ in length
- longer MT -> shortened
- shorter MT -> elongated
MT dynamic facilitates what?
chromosome movement and alignment (as well as motors)
shortening and elongation of microtubules occur primarily how?
by loss or gain of ab-tubulin subunits at the + end of the microtubule
***while attached at the kinetochore!!!!
mitotic spindles at metaphase: microtubule populations
- chromosomes
- astral spindle microtubules
- pericentriolar material
- chromosomal (kinetochore) spindle fibers
- polar spindle microtubules
chromosomes at metaphase
metaphase plate
astral spindle microtubules at metaphase
position the spindle apparatus
chromosomal (kinetochore) spindle fibers at metaphase
exerts pulling force at the kinetochore
- move the chromosome, a molecular tug of war
polar spindle microtubules at metaphase
maintain integrity of the spindle
pericentriolar material at metaphase
organize and nuclear microtubules
how does a cell "know" whether all chromosomes are attached properly and aligned at the metaphase plate?
before anaphase occurs, the spindle assembly checkpoint must be satisfied
spindle assembly checkpoint
unattached kinetochore assembles a complex to delay anaphase ->
mitotic checkpoint complex ->
kinetochore binds to a MT bundle ->
ubiquitylation ->
two pathways from ubiquitination: either lead to active or inactive
mitotic checkpoint complex
keeps APC active
AurB
helps sense and correct attachments
what happens when the kinetochore binds to a MT bundle?
it kicks off Mad2 complex
- it gets moved away by dynein-1
inactive pathway of ubiquitylation after kinetochore binds MT bundle
ubiquitin + cyclin B + Cdk1
-(degradation)->
inactive
active pathway of ubiquitylation after kinetochore binds MT bundle
ubiquitin + securin + separase
-(degradation)->
active ->
degradation of cohesin ->
anaphase triggered
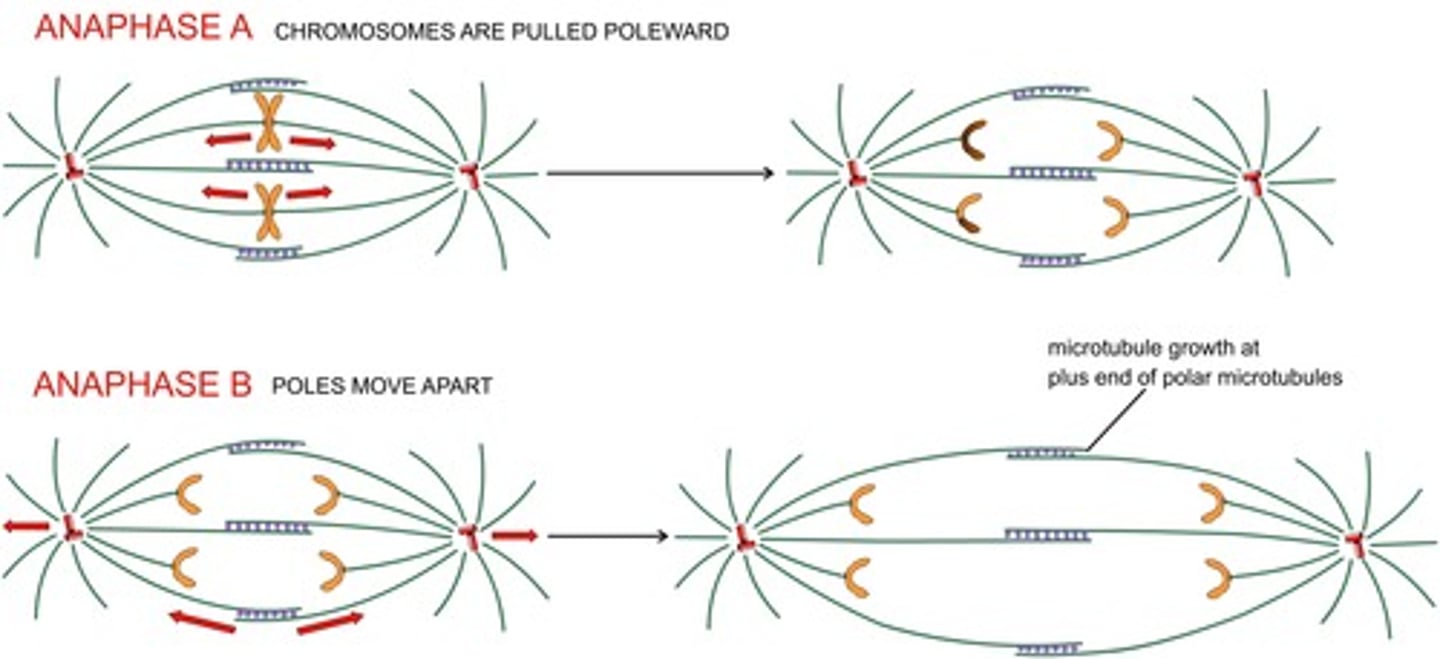
what moves the chromatid pairs?
microtubule depolymerization powers chromatin movement
disassembly-force model
- MT depolymerization generates sufficient force to pull a chromatid
- remember, chromatids are tethered to the microtubule + end
anaphase A
- shortening of "chromosomal spindle" at both + and - ends
- movement of the chromosomes toward poles
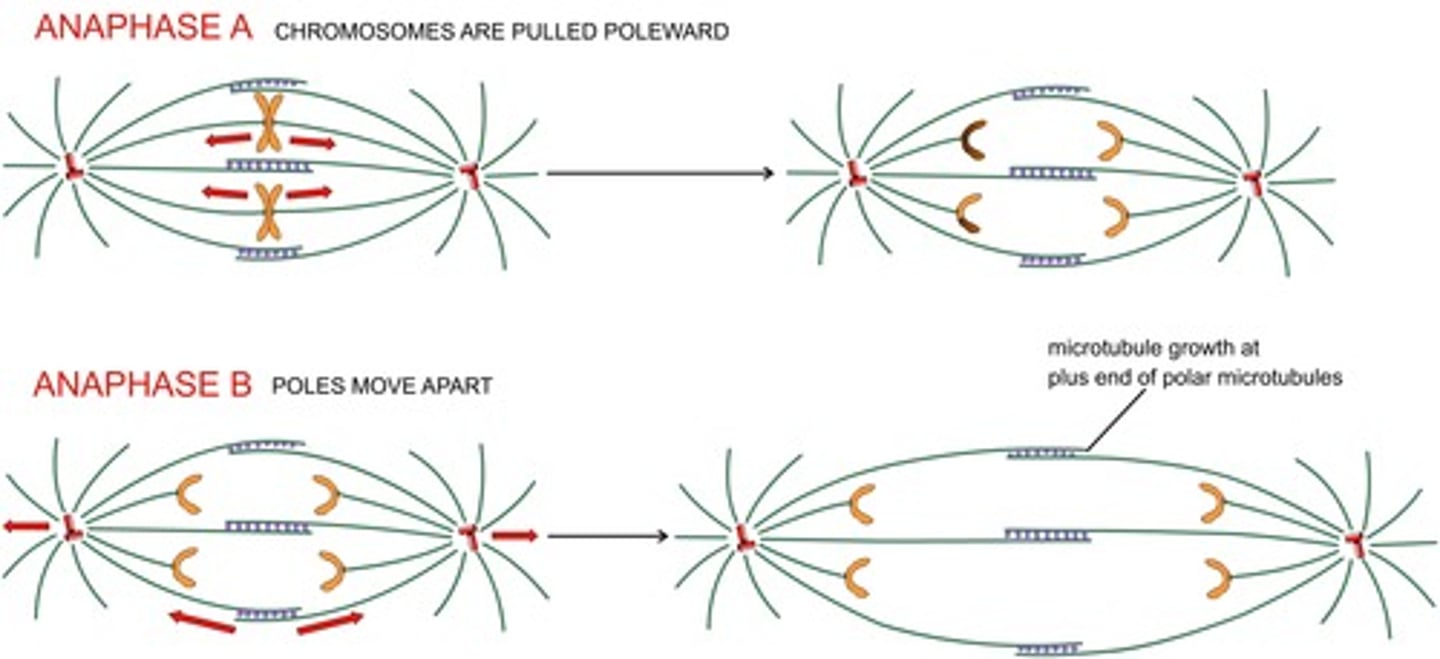
anaphase B
movement of the poles apart = elongation of the polar spindle
If you use CRISPR-Cas9 to delete Mad2, what would you likely observe?
abnormal chromosome segregation
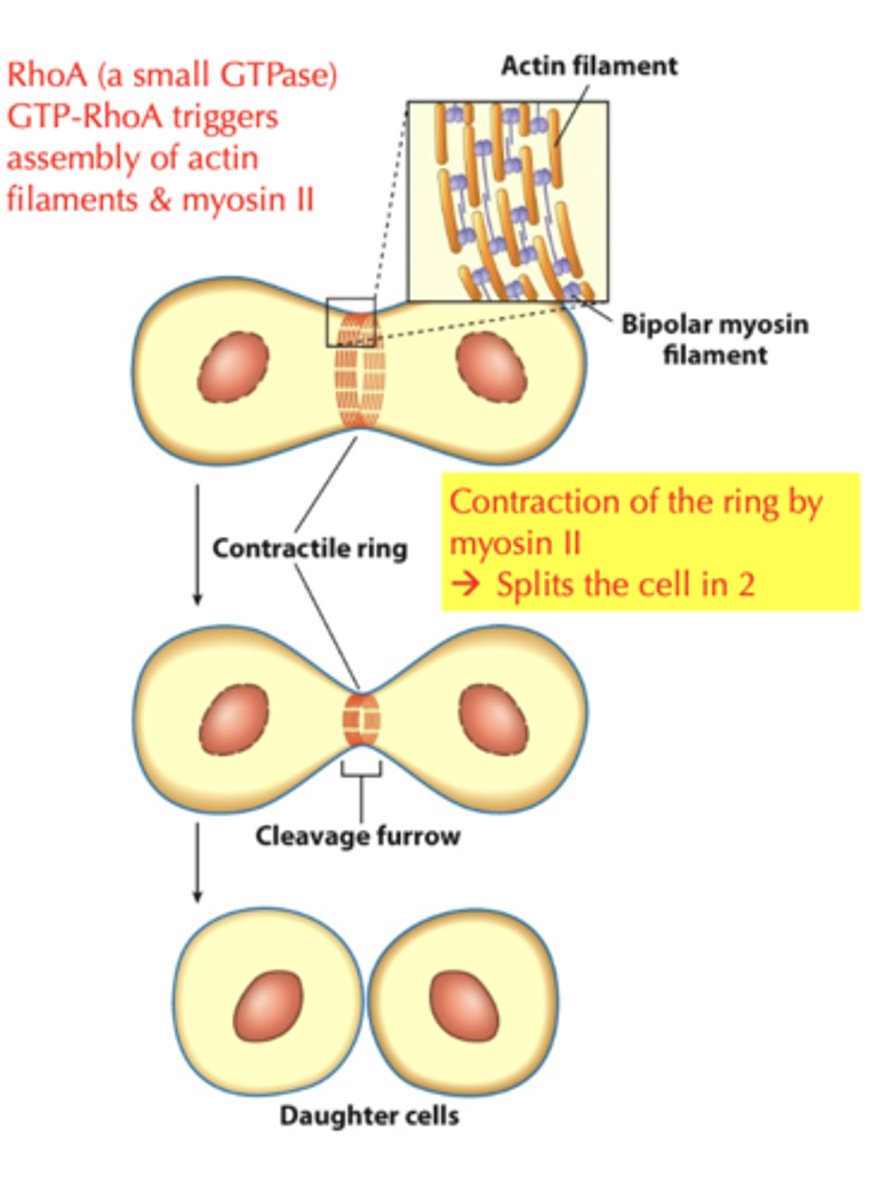
contractile ring during cytokinesis
- cell divides into 2 daughter cells, division of the cytoplasm
- actin filaments are concentrated within the cleavage furrow
- LOOK AT DIAGRAM!!!!
cell migration is required for many processes in higher vertebrates
- tissue and organ development
- formation of blood vessels
- development of axons
- wound healing
- protection against infection, etc
cell migration also contributes to what?
the spread of cancerous tumors- metastasis
drugs that disrupt dynamic actin based activities
actin mediated processes rapidly stop when cells contain one of these compounds
- chytochalasin
- phalloidin
- latrunculin
chytochalasin
- derived from mold
- blocks the barbed ends of actin microfilaments and allows depolymerization at the pointed end
phalloidin
- obtained from a poisonous mushroom
- binds to intact actin filaments and prevents their turnover
latrunculin
- obtained from a sponge
- binds to free monomers and blocks their incorporation into the polymer
cell motility is required for what?
bone healing
fibroblast
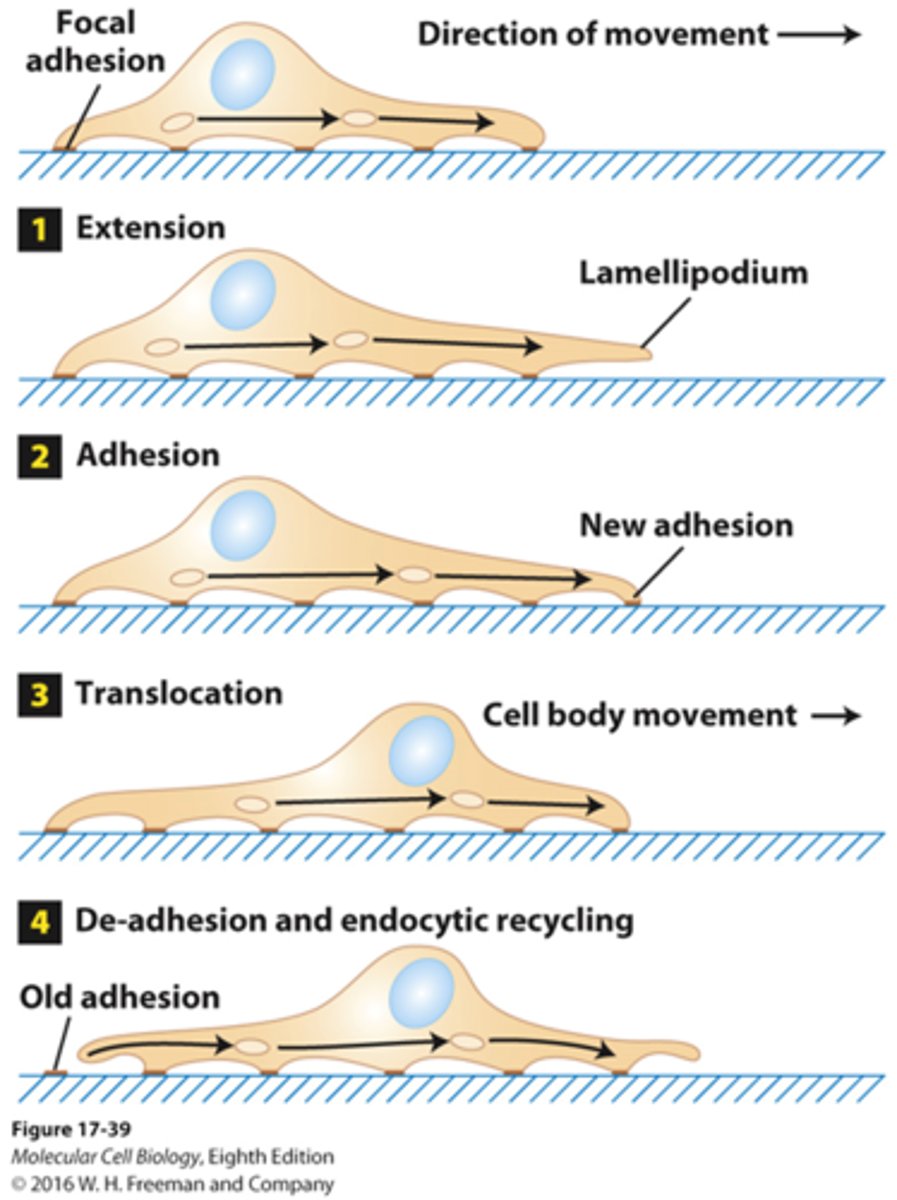
what is the leading edge in fibroblast movement?
lamellipodium
- no motors directly involved
- accomplished through F-actin polymerization dynamics
- uses actin-binding proteins and treadmilling
lamellipodium
- flat and very thin
- provides new anchorage points
- provides protrusive force
- actin treadmilling is critical
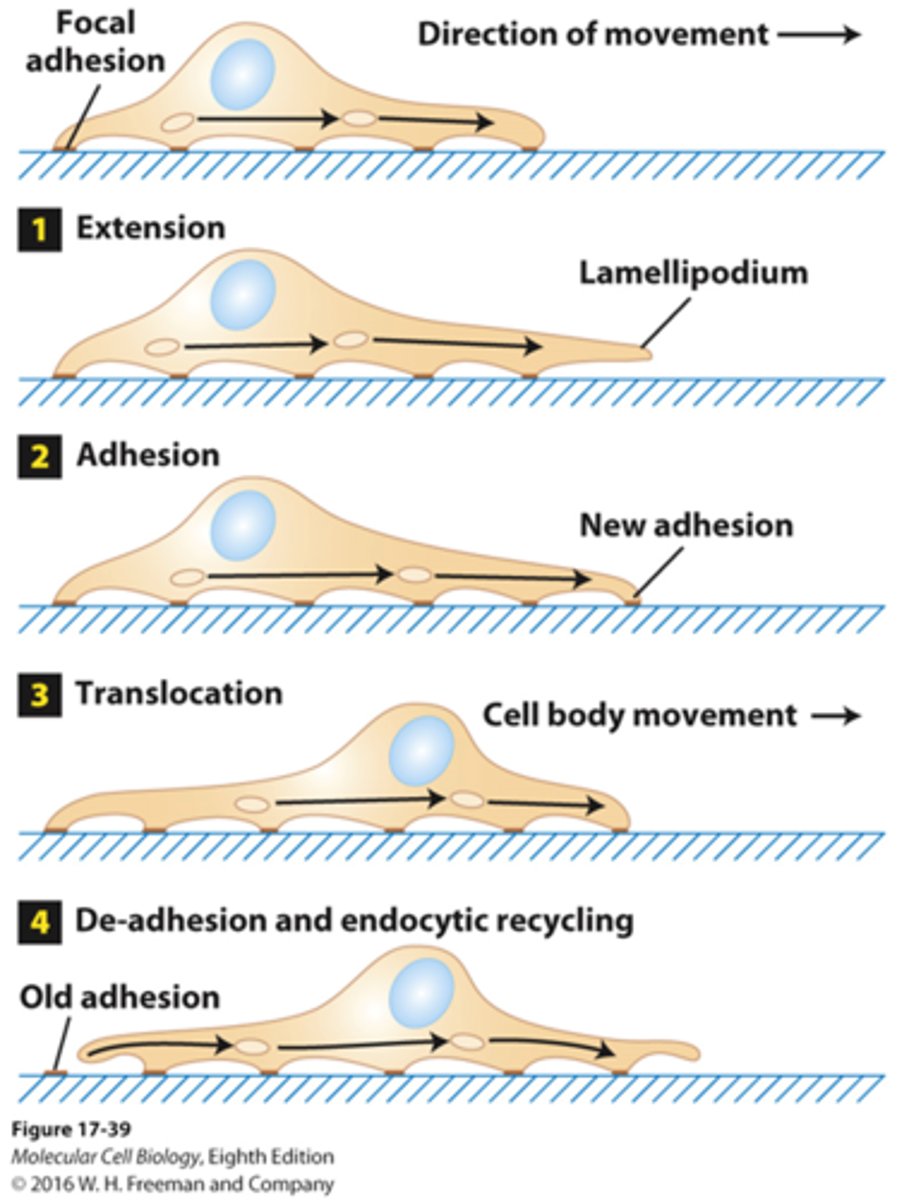
fibroblast movement
extracellular signals -> intracellular F-actin reorganization and assembly that protrudes the membrane forward -> movement
WASP
- Wiskott-Aldrich syndrome
- NO functional WASP
-> cell cannot respond to chemotatic signals
-> cells have migration defect
-> crippled immune system
Arp2/3 complexes
nucleate F-actin and form "Y-branches"
critical parts of cell migration
- protrusion
- adhesion
- pulling
- pushing
vinculin
focal adhesions
actin-based infection
some intracellular bacteria use F-actin polymerization for their movement and infection
Listeria movement
directly proportional to F-actin polymerization
bacterial protein in listeria movement
ActA
- ActA on listeria stimulates nucleating activity of Arp2/3
host proteins in listeria movement
- actin
- Arp2/3
- cofilin
- capping protein
- profilin
- and steady supply of ATP
profilin in listeria movement
stimulates actin to bind ATP
listeria actin treadmilling is the same as in lamellipodia
- some endosomes in cells use this actin polymerization machine to move
- the actin "tail" forms by filament polymerization on the bacteria surface
-> this begins to "push" it, the "tail" length is maintained via treadmilling
it was found that listeria infection could NOT be spread from one cell to its neighbors on a petri dish in the presence of cytochalaisin (an inhibitor of actin polymerization). this suggested what?
actin polymerization is required for listeria infection
apoptosis
programmed cell death
- normal process that is unique to animal cells
- proteins are specifically degraded
- cell shape changes (overall shrinkage in volume)
- membrane blebbing
- cell detachment
- DNA fragmentation
- highly regulated dissassembly
- energy dependent
necrosis
- generally follows some type of physical trauma or chemical insult
- special necrosis can also occur as a regulated and programmed process (necrop-tosis), although much less orderly in nature
- characterized by:
-> swelling of the cell and its internal membranous organelles
-> membrane breakdown
-> leakage of cell contents into the extracellular space, resulting in inflammation
apoptosis in normal physiology- embryonic development
- around 330 billion cells are replaced daily in our bodies
- over ~3 months, this equals the number of cells in our entire body
examples of apoptosis during development
- a limb bud is transformed into a real foot/hand by interdigital apoptosis
- apoptosis used to create many hollow structures in our bodies (ducts in breast)
- the tadpole loses its tail to become a toad by apoptosis
- males lose their female side (Mullerian duct that forms uterus and ovaries) and females lose their male side (Wolfian duct that forms epididymis and seminal vesicles) by apoptosis
enzymes termed caspases are involved in apoptosis
caspases are proteases that cleave many proteins:
- kinases: FAK, PKB, PKC, and Raf1 (more)
- lamins
- intermediate filaments, actin, tubulin, and gelsolin
- caspase cleavage of an inhibitory protein called ICAD. this allows CAD to be active
CAD
caspase activated DNase
pro-caspases
caspases are present in every cell as inactive enzymes called pro-caspases
caspases are activated in cascades
the cascades lead to extensive protein degradation via proteolysis
- initiators can activate the executioners
- cleavage of cytosolic protein
- cleavage of nuclear lamin
- some "executioner" caspases can activate other "executioner" caspases
initiator and executioner caspases
- initiator caspases: 2, 8, 9, 10
- executioner caspases: 3, 6, 7
zVAD-fmk
a peptide "drug" called zVAD-fmk inhibits all caspases
- many other selective caspase inhibitors also exist
apoptosis plays a role in various human diseases
- involved in neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's
-> elimination of essential neurons during disease progression
- in type 1 diabetes, cells are abnormally killed via apoptosis
- in cancers, where damaged cells would otherwise be killed, they instead evade and escape the ability to die, allowing their evolution
apoptosis can be triggered by what?
apoptosis during development appears to use both pathways:
- external stimuli
- internal stimuli
extrinsic apoptosis pathway
receptor mediated
- triggered by external stimuli such as certain cytokines
intrinsic apoptosis pathway
mitochondria-mediated
- triggered by internal stimuli such as abnormalities in the DNA
cytokines
proteins secreted by cells, especially in the immune system