Equilibrium: Keq & Le Chatelier's Principle
0.0(0)
0.0(0)
New
Card Sorting
1/17
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
1
New cards
reversible reaction
the conversion of reactants to products and the conversion of products to reactants occur simultaneously
2
New cards
conditions for equilibrium
1. systems must be closed
2. equilibrium is a dynamic process
3. rates of forward and reverse reactions are equal
4. the amounts of reacts and products don’t have to be equal, but, after equilibrium is attained the amounts of reactants and products will be equal
3
New cards
equilibrium constant
the ratio of the mathematical product of the concentrations of the products of a reactions to the mathematical product of the concentrations of the reactants of the reaction
4
New cards
how do you know a reaction is at equilibrium
1. the rate of the forward reaction is equal to the rate of the reverse reaction
2. concentration of the products and reactants are constant (concentration doesn’t change)
5
New cards
homogenous equilibrium
ALL substances in the reaction exist in the same state
6
New cards
heterogenous equilibrium
substances exist in different states
7
New cards
equilibrium constant expression
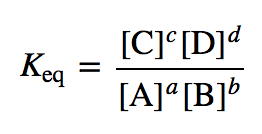
8
New cards
\[ \]
M or mol/L
9
New cards
when writing Keq expressions
ONLY (g) and (aq) reactants/ products are included
EXCLUDED: (s) and (l)
EXCLUDED: (s) and (l)
10
New cards
when writin Keq expressions coefficents become
exponents in the Keq expression
11
New cards
Le Chatelier’s Principle
when stress is applied to a system at equilibrium, the system will react in such a way to relive the stress
12
New cards
when there is an increase in the concentration of a substance the system will
shift to consume the increasing concentration
13
New cards
when there is a decrease in the concentration of a substance by removing some amount the system will
shift to the side to replace/ produce more of the substance being removed
14
New cards
when there is an increase in pressure the system will shift to the side where
there is a SMALLER number of GASEOUS moles
15
New cards
when there is a decrease in pressure the system will shift to the side where
there is a GREATER number of GASEOUS moles
16
New cards
when there is an increase in temperature the system will shift to the side where
the added heat is consumed or the ENDOthermic reaction
17
New cards
when there is a decrease in temperature the system will shift to the side where
the heat will be generated or the EXOthermic reaction
18
New cards
endothermic reaction symbol
exothermic reaction symbol
exothermic reaction symbol
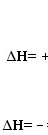
Explore top notes
Chapter Fifteen: Treatments for Schizophrenia and Other Severe Mental Disorders
Updated 911d ago