BMS 704 - Exam #1
1/160
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
161 Terms
What are the cellular constituents that are targets of cell injury?
cell membrane (includes cell, mitochondria & nucleus), ATP production (mitochondria), protein synthesis & genetic apparatus (nucleus)
What cells are sensitive to hypoxia?
cardiac myocytes, neurons, renal proximal tubular epithelium (kidneys —> has the highest concentration of the sodium/potassium pump), hepatocytes (liver) & endothelium (tissue)
What are the characteristics of reversible cell injury?
surface blebbing, mild swelling of cells/organelles, ribosomes detach, & chromatin clumping
What are the plasma membrane alterations that occurs in reversible cell injury?
surface blebbing, surface blunting, creation of myelin figures, distortion/loss of microvilli & loosening of intracellular attachments
What are the mitochondrial changes that occurs in reversible cell injury?
swelling, rarefaction (decreased density) & small phospholipid-rich amorphous (calcium) densities
What happens due to the dilation of the endoplasmic reticulum during reversible cell injury?
detachment and disaggregation (breakdown) of polysomes
What are the nuclear alterations that occurs in reversible cell injury?
disaggregation (breakdown) of granular and fibrillar elements
What are the characteristics of irreversible cell injury?
marked swelling, lysosome disruption, amorphous (calcium) deposits in mitochondria, membrane disruption, nuclear changes (ex. pyknosis) & more myeline fingers
What are the major mechanisms of cell damage?
decrease in ATP, mitochondrial damage, increase of intracellular calcium, increase in reactive oxygen species (ROS), membrane damage & protein misfolding/DNA damage
What are the causes of a decrease in ATP?
hypoxia, decreased nutrients, mitochondrial damage & toxins
What are the downstream effects of a decrease in ATP?
failure of the Na/K & Ca pump, ribosomes detach leading to a decrease in proteins, failure of glycolysis & decrease in pH & an accumulation of misfolded proteins
What are the causes of mitochondrial damage?
increased amount of intracellular calcium, free radicals/ROS, hypoxia & toxins
What are the downstream effects of mitochondrial damage?
decrease production of ATP —>release of protons out of the mitochondrial membrane (mitochondrial permeability transition) —> loss of membrane potential —> inability to generate ATP —> necrosis
or
release/leakage of cytochrome c & other pro-apoptotic proteins —> apoptosis
What are the causes in an increase of intracellular calcium?
hypoxia & toxins
What are the downstream effects of an increase of intracellular calcium?
activation of phospholipases (decreases phospholipids)& proteases (disrupts the membrane & cytoskeletal proteins —> membrane damage
activation of endonucleases —> nuclear damage
activation of ATPase &as well as an increase in mitochondrial permeability transition —> decrease in ATP
What are the causes of an increase in reactive oxygen species (ROS)?
inflammation, decreased removal/scavenging, radiation & drugs
What are the downstream effects of an increase in reactive oxygen species (ROS)?
reaction with fatty acids —> oxidation —> generation of lipid peroxidases —> disruption of plasma membrane organelles
reaction with proteins —> oxidation —> loss of enzymatic activity & abnormal folding
reaction with DNA —> oxidation —> mutations & breaks
How are free radicals removed?
SOD (mitochondria) —> converts O2 into H2O2
glutathione peroxidase (mitochondria) —> converts OH into H2O2 into H2O + O2
catalase (peroxisomes) —> converts H2O2 into H2O2 + O2
What are the causes of membrane damage?
free radical generation & ATP depletion (not good protein structure maintenance)
What are the downstream effects of membrane damage?
mitochondrial membrane - opens mitochondrial permeability transition pore —> decreased ATP & apoptotic triggering proteins
plasma membrane - influx of Na+, water & Ca2+ & loss of K+/other cellular substances
lysosomal membrane - leakage of enzymes that digest protein, DNA, RNA & glycogen
What are the causes of protein misfolding & DNA damage?
mutations
What are the downstream effects of protein misfolding & DNA damage?
internal matrix - loss of enzyme function
cell surface matrix - decrease apoprotein leads to a fatty liver
structural matrix - cell rupture
signaling function -loss of cell receptor function
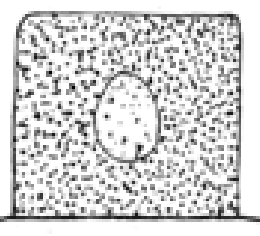
Define karyorhexis.
the pyknotic or partially pyknotic nucleus undergoes fragmentation (rhexis —> break a part)
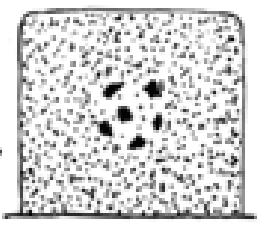
Define pyknosis.
characterized by nuclear shrinkage and increased basophilia
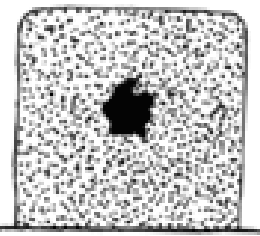
Define karyolysis.
the basophilia of the chromatin may fad, a change that reflects DNAase activity
What is white muscle disease?
ROS induced injury leading to necrotizing myocarditis & myositis produced in animals
caused by a deficiency in vitamin E and selenium
What are the forms of necrosis?
liquefactive, caseous & coagulative
What are the characteristics of coagulative necrosis?
architecture is preserved, firm & enzyme damage
What are the causes of coagulative necrosis?
ischemia (except CNS), bacterial exotoxins & chemical toxins
What are the characteristics of caseous necrosis?
cheese-like & loss of architecture
What are the causes of caseous necrosis?
tuberculosis/Mycobacteria spp & Cornybacterium
What is dry gangrene?
coagulation necrosis secondary to infarction followed by mummification
happens on the extremities, tissues dry out and bacteria can’t grow
What is moist/wet gangrene?
necrotic tissue, has further degradation (breakdown) by bacteria of rot
What is gas gangrene?
anaerobic bacteria such as Clostridium sp
grow and release toxins and gas in dead tissue
What are the five classical characteristics of inflammation?
calor, rubor, tumor, dolor & functio laesa
What is calor?
heat
What is rubor?
redness
What is tumor?
swelling
What is dolor?
pain
What is functio laesa?
loss of function
What is the time course of acute inflammation?
minutes to days
What are the cellular constituents of acute inflammation?
neutrophils
What is the vascular characteristics of acute inflammation?
fluid and plasma leakage
What are the features of interstitium for acute inflammation?
What is the time course for chronic inflammation?
days to years
What are the cellular constituents of chronic inflammation?
lymphocytes & macrophages
What are the vascular characteristics of chronic inflammation?
vascular proliferation
What are the features of interstitium for chronic inflammation?
fibrous tissue proliferation (scar)
What are the names of the process of fluid leakage in acute inflammation?
transudate and exudate
What is the mechanism for transudate vascular leakage in acute inflammation?
increased hydrostatic pressure (venous outflow obstruction) —> fluid leakage —> decreased colloid osmotic pressure (decreased protein synthesis) —> increased protein loss
low protein content & few cells
What is the mechanism for exudate vascular leakage in acute inflammation?
fluid & protein leakage —> vasodilation & stasis (inflammation) —> increased interendothelial spaces (inflammation) —> wider gaps/vascular permeability
high protein content & may contain some white & red cells
Where does the majority of fluid leakage, leukocyte transmigration & hemorrhage occur?
capillaries & postcapillary venules
What results from an increase in vascular permeability?
retraction of endothelial cells & endothelial injury
What are the causes for an increase in vascular permeability leading to retraction of endothelial cells?
induced by histamine, nitric oxide & other mediators
What is the speed for an increase in vascular permeability leading to retraction of endothelial cells?
rapid & short lived (minutes)
Where does an increase in vascular permeability leading to retraction of endothelial cells occur?
venules
What are the causes for an increase in vascular permeability leading to endothelial injury?
caused by burns & some microbial toxins
What are the speed for an increase in vascular permeability leading to endothelial injury?
rapid & can be long lived (hours-days)
Where does an increase in vascular permeability leading to endothelial injury occur?
arterioles, capillaries & venules
What happens to the cell size during necrosis?
enlarged (swelling)
What happens to the nucleus during necrosis?
pyknosis —> karyorrhexis —> karyolysis
What happens to the plasma membrane during necrosis?
disrupted
What happens to the cellular contents during necrosis?
enzymatic digestion; may leak out of the cell
Is there adjacent inflammation during necrosis?
yes, frequently
Is there a physiologic or pathologic role in necrosis?
invariably pathologic —> culmination of irreversible cell injury
What happens to the cell size during apoptosis?
reduced (shrinking)
What happens to the nucleus during apoptosis?
fragmentation into nucleosome size fragments
What happens to the plasma membrane during apoptosis?
intact; altered structure, especially orientation of lipids
What happens to the cellular contents during apoptosis?
intact; may be released in apoptotic bodies
Is there adjacent inflammation during apoptosis?
no
Is there a physiologic or pathologic role in apoptosis?
often physiologic —> eliminating unwanted cells
sometimes pathologic after some forms of cell injury, especially DNA damage
What are the anti-apoptotic proteins found in the intrinsic pathway?
Bcl-2, Bcl-x & Mcl-1
What do anti-apoptotic proteins do in the intrinsic pathway?
stops the cell from going through apoptosis
control the mitochondrial permeability
increase permeability to release pro-apoptotic molecules
growth factors & survival signals increase anti-apoptotic proteins
What are the pro-apoptotic proteins found in the intrinsic pathway?
BH-3 (Bim, Bid & Bad)
What do the pro-apoptotic proteins do in the intrinsic pathway?
senses damage to lead to apoptosis
antagonize & decrease Bcl-2 (anti-apoptotic protein) synthesis
activate bax & bak
What is the mechanism of Smac/DIABLO in the intrinsic pathway for apoptosis?
cytochrome c binds to Apaf-1 which activates caspase-9, however IAPs block caspase activation to keep the cells alive but Smac/DIABLO binds to and neutralizes the IAPS to activate the executioner caspases to then induce apoptosis
What is the mechanism of the intrinsic pathway for apoptosis?
cytochrome C is released and binds/activates Apaf-1 on the pro-caspase-9 molecule and activates the caspase, the active caspase-9 then activates the executioner caspases that then induces apoptosis
What is the mechanism of the extrinsic pathway for apoptosis?
TNF (death) receptor is bound to Fas where Fas then binds to FasL (on self reactive T cells), 3 Fas proteins are brought together to form the binding site for FADD which activates pro-caspase 8 or 10
What inhibits the extrinsic pathway for apoptosis?
FLIP protein inactivates this pathway by binding to procaspase-8, inhibiting its activation
What are the cellular events of acute inflammation?
leukocyte recruitment where there is margination, partial adhesion and rolling along the endothelium of the post-capillary venules where there is then firm adhesion to the activated endothelium where cytokines and chemokines mediated the surface expression and binding avidity of the adhesion molecules, there is then transmigration between the endothelial cells (post-capillary venules) to lead to migration into the interstitial tissues towards a chemotactic stimulus such as: chemotaxis that will move along a chemical gradient, exogenous sources like bacterial peptides, endogenous sources like cytokines/chemokines (IL-8), C5a and LTB4
What are the leukocyte receptors for p-selectin?
Sialyl-Lewis X & PSGL-1
What is the major role of p-selection?
rolling (PMN, monos & lymphs)
What is the leukocyte receptor for e-selectin?
Sialyl-Lewis X
What is the major role of e-selectin?
rolling & adhesion to activated endothelium (PMN, monos & T-cells)
What are the leukocyte receptors for ICAM-1?
CD11/CD18 (integrins)
What is the major role of ICAM-1?
adhesion, arrest & transmigration (all)
What are the leukocyte receptors of VCAM-1?
alpha4beta1 integrins
What is the major role of VCAM-1?
adhesion (eos, monos & lymphs)
What is the leukocyte receptor of GlyCam-1?
L-selectin
What is the major role of GlyCam-1?
lymphocyte homing & PMN/mono rolling
What is the leukocyte receptor of PECAM?
CD31
What is the major role of PECAM?
leukocyte migration through endothelium
What is rolling in acute inflammation mediated by?
selectins (weak)
What is firm adhesion in acute inflammation mediated by?
integrins
What does IL-1 & TNF do in acute inflammation?
induce endothelial expression of p-selectin, e-selectin and integrin ligands (ICAM-1 & VCAM-1)
What do chemokines do in acute inflammation?
activate integrins on white blood cells to form stronger bonds with their ligand (firm adhesion)
What do the receptors for opsonins do during leukocyte activation?
causes phagocytosis where a particle is coated with opsonin to become a target through opsonization
Fc and complement receptors bind to IgG antibodies, complement proteins (C3b breakdown products) & lectins (mannan-binding lectin)
What do the receptors for cytokines do during leukocyte activation?
natural killers (NK) cells & T cells secrete interferon-gamma
What is the major macrophage-activating cytokine in leukocyte activation?
interferon-gamma
How do reactive oxygen species (ROS) aid in the killing and degradation of bacteria?
during phagocytosis NADPH oxidase oxidizes NADPH to produce free radicals to kill the bacteria
myeloperoxidase enzyme in neutrophils combine with H2O2 and Cl- to produce hypochlorite (bleach) to destroy microbes through halogenation or oxidation of proteins & lipids