rate law
0.0(0)
Card Sorting
1/14
There's no tags or description
Looks like no tags are added yet.
Last updated 3:40 PM on 2/26/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
1
New cards
0th order rate law
rate = k; units: mol/L*s
2
New cards
1st order rate law
rate = k[A]; units: 1/s
3
New cards
2nd order rate law
rate = k[A][B]; units: L/mol*s
4
New cards
0th order integrated rate law
[A]final = -kt + [A]initial
5
New cards
1st order integrated rate law
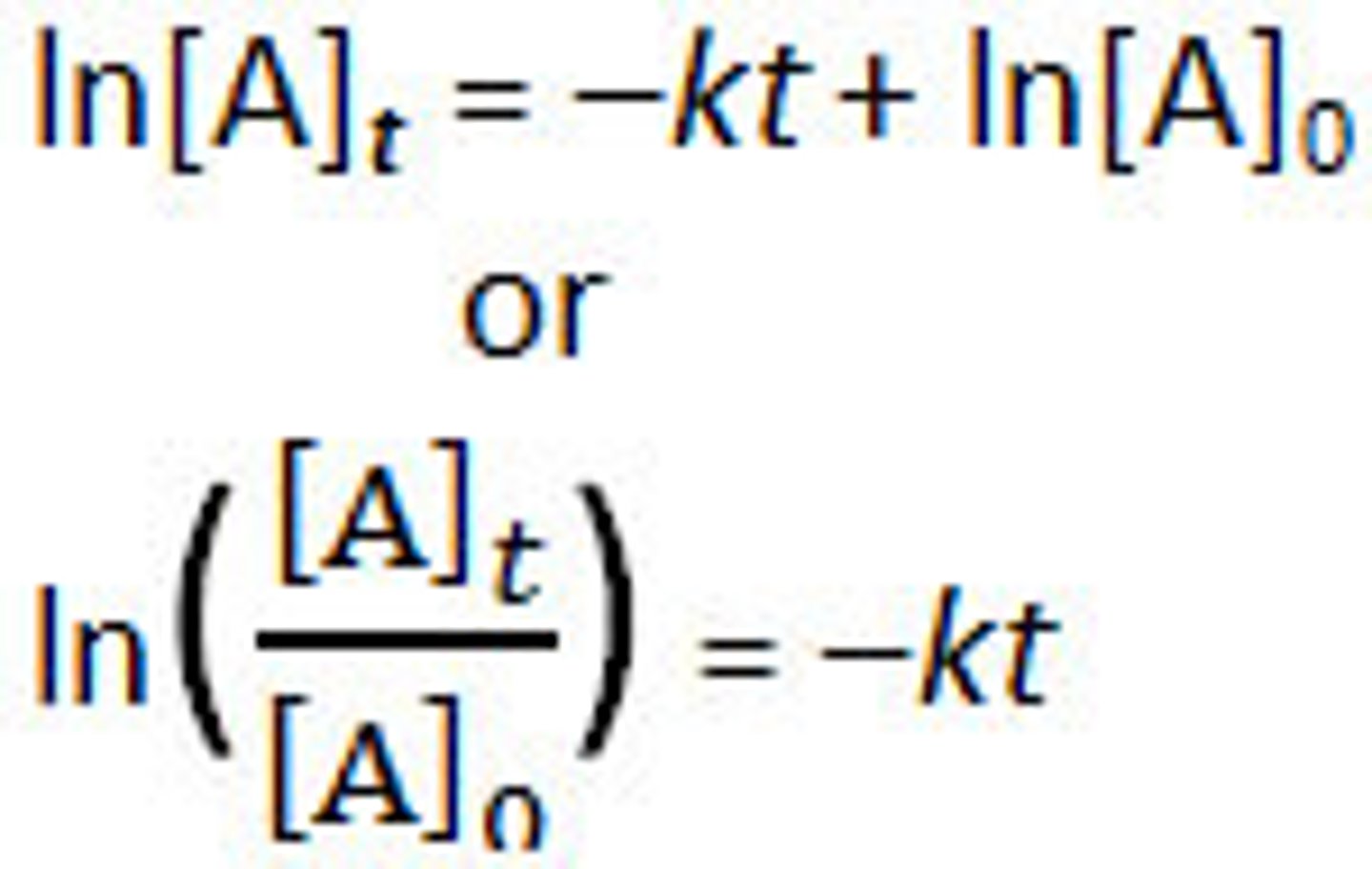
6
New cards
2nd order integrated rate law
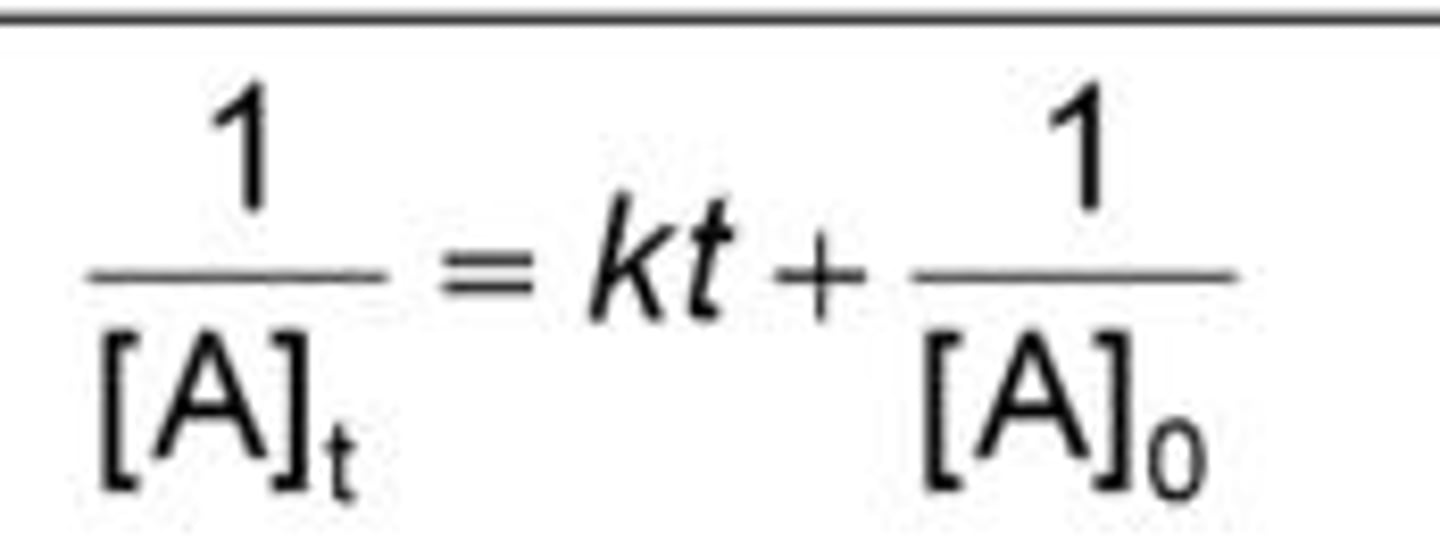
7
New cards
0th order half-life
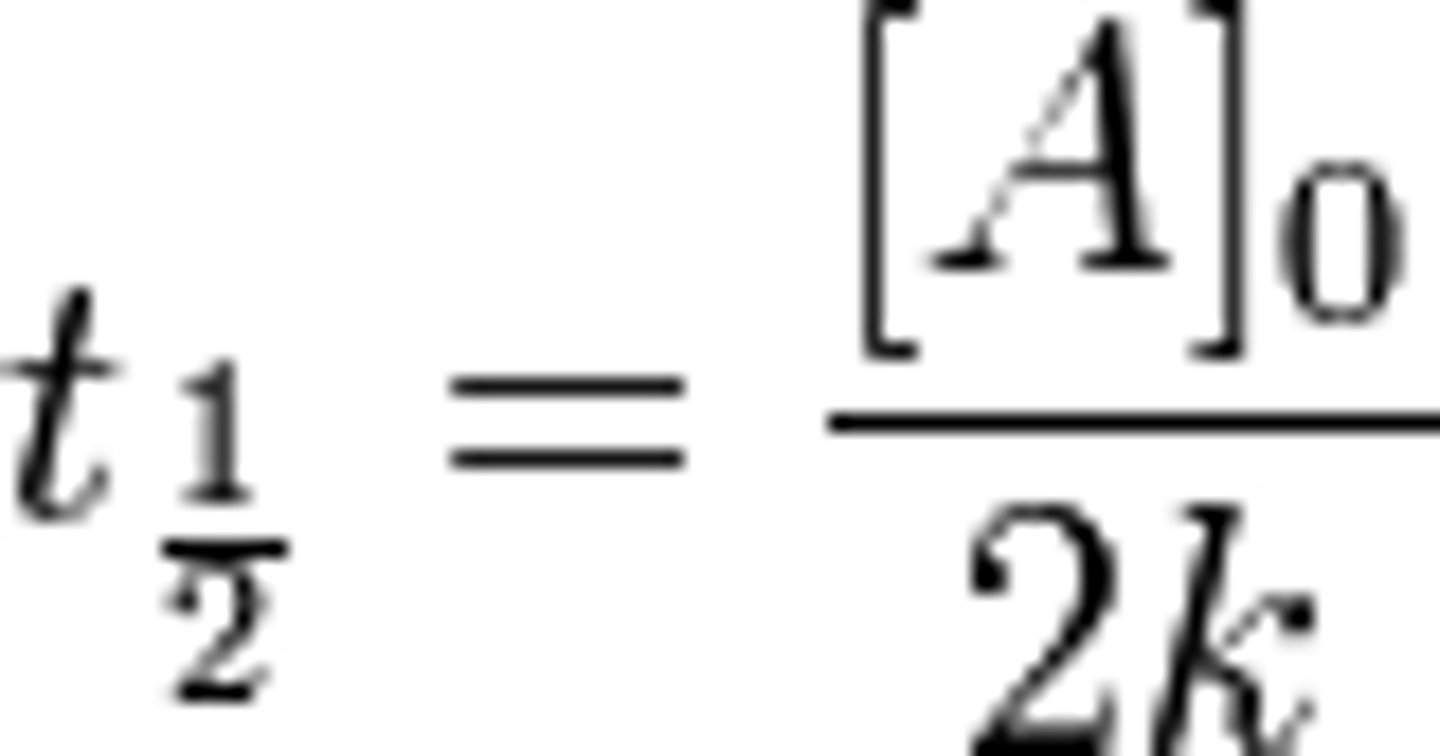
8
New cards
1st order half-life
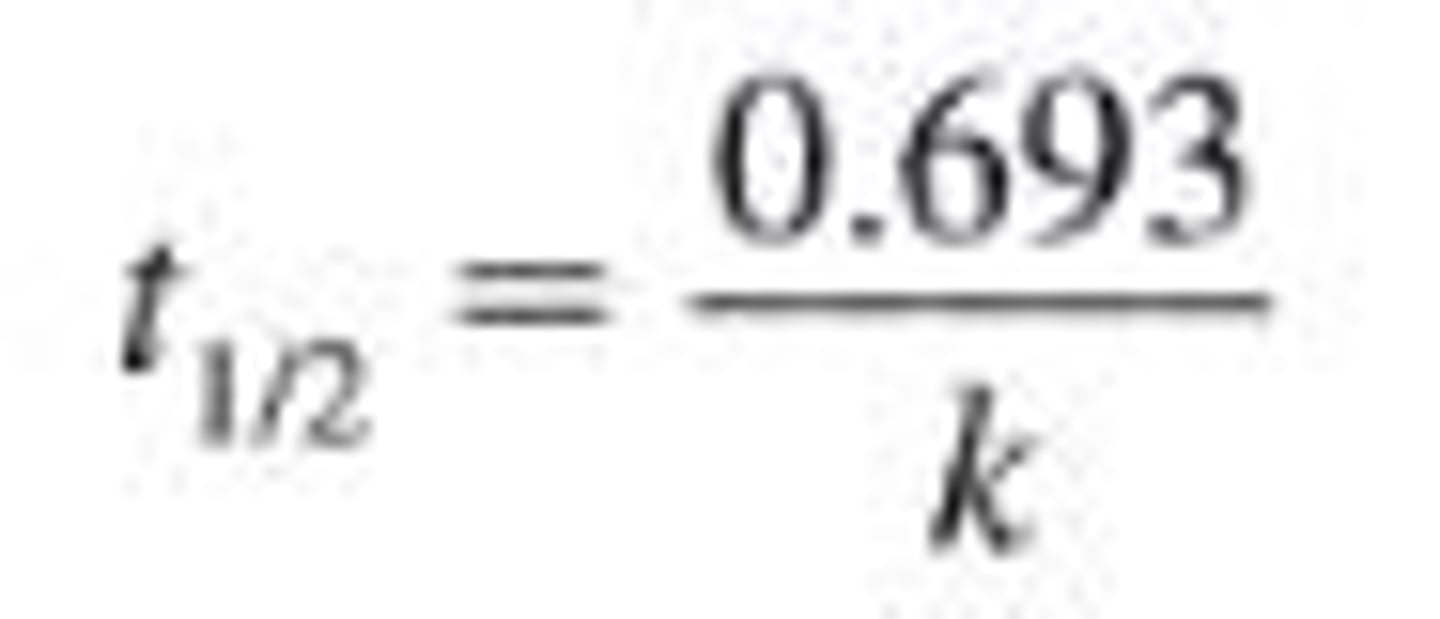
9
New cards
2nd order half-life
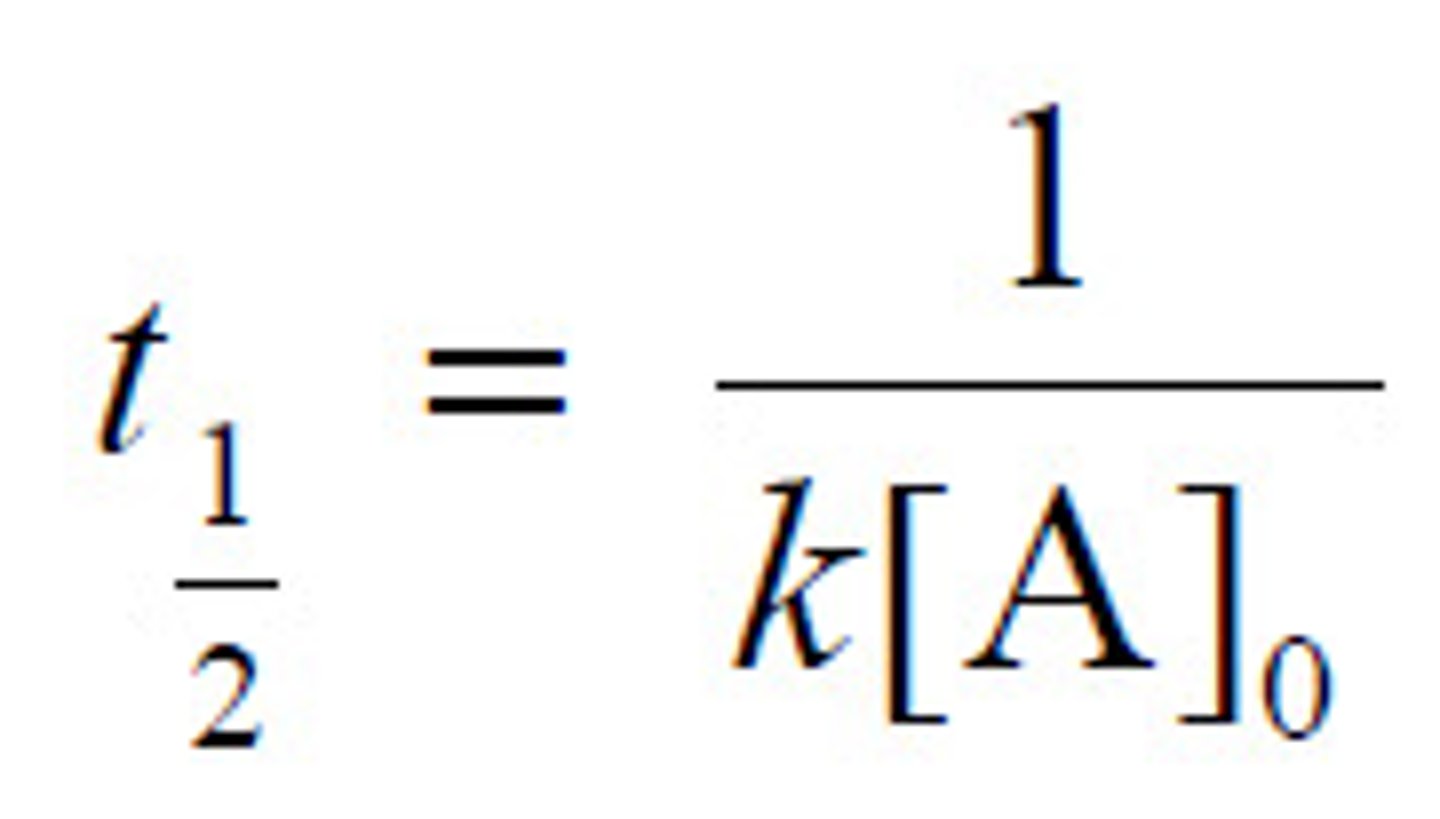
10
New cards
1st order graph
ln[A] vs time
![<p>ln[A] vs time</p>](https://knowt-user-attachments.s3.amazonaws.com/a9912415-eb64-4b1d-84cb-6b6ff5820d0f.png)
11
New cards
2nd order graph
1/[A] vs time
![<p>1/[A] vs time</p>](https://knowt-user-attachments.s3.amazonaws.com/cd4979e5-55cb-49ad-aea9-e957ec397f8b.png)
12
New cards
0 order graph
[A] vs time
![<p>[A] vs time</p>](https://knowt-user-attachments.s3.amazonaws.com/e79e3d68-6d14-4065-81c2-b4422c231688.jpg)
13
New cards
1st order half life is
constant
14
New cards
2nd order half life is
NOT constant, each successive half life doubles
15
New cards
0 order half life is
NOT constant, each successive half life is 1/2