Fluid and electroytes part 2 video
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
What are electrolytes?
Substances that create an electrically conducting solution in water by dissociating into ions.
How do electrolytes function when dissolved in water?
They form ions that conduct electrical current.
What is the role of electrolytes in the body regarding osmosis?
Controlling the movement of water between body fluid compartments.
Why are electrolytes crucial for carrying electrical current in the body?
They are essential for membrane potential, including:
resting membrane potential
graded potential
action potential
What function do electrolytes serve as cofactors for?
Enzymes, ensuring proper enzymatic function.
Which ions are typically formed by electrolytes upon entering body fluids?
Sodium, potassium, calcium, magnesium, and chlorine.
What distinguishes anions from cations?
Anions are negative ions, while cations are positive ions.
How are ions with multiple charges per atom measured in body fluids?
In milliequivalents to account for the number of charges per atom.
Where are ions typically found in the body, based on the provided chart insights?
Both inside and outside of cells.
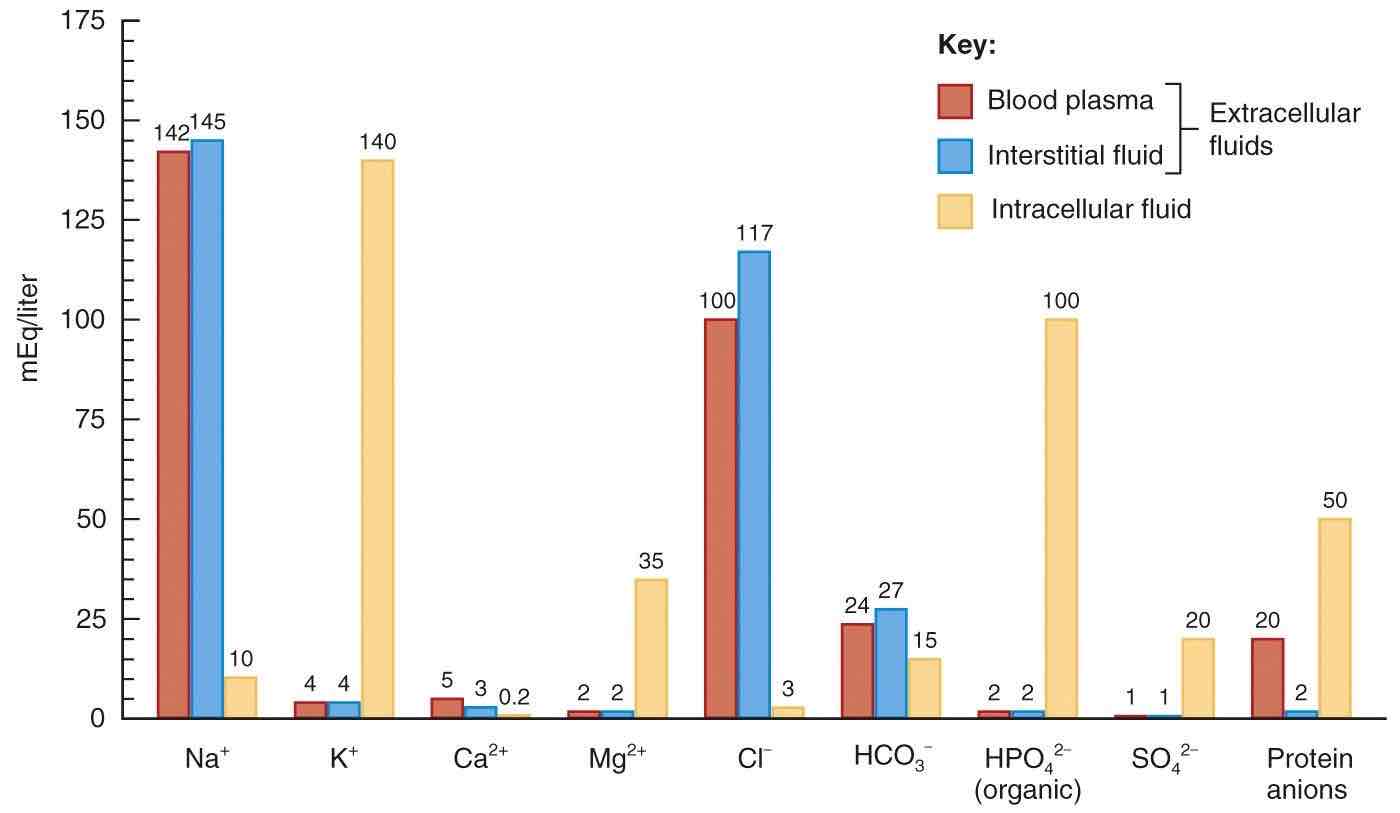
Which bodily fluids share similarities in terms of ion presence according to the chart?
Blood plasma and interstitial fluid.
What is the major difference in the distribution of sodium and potassium between extracellular and intracellular fluids?
Extracellular fluid contains about 10 times more sodium than intracellular fluid, while potassium is mainly found inside cells.
Why is the distribution of sodium and potassium crucial for the function of the sodium-potassium pump?
Cells maintain higher concentrations of sodium outside and higher concentrations of potassium inside, which is essential for membrane potentials and the function of the sodium-potassium pump.
Where is calcium most abundant in the body despite being less prevalent in fluids?
Calcium is most abundant in bone tissue despite being less prevalent in fluids.
What essential roles does calcium play in plasma and interstitial fluid?
Calcium in fluids plays essential roles in blood clotting, neurotransmitter release, and the electrical excitability of nervous and muscle tissue.
Where is around half of the body's magnesium stored, and what is its main function as a cofactor?
Around half of the body's magnesium is stored in bone tissue, and it acts as a crucial cofactor for numerous enzymes, regulating their activity and participating in various cellular processes.
Why is chloride essential for the body, and with which ion does it commonly enter the body?
Chloride is essential for cotransport of other ions across membranes and helps neutralize the effects of certain ions moving between fluid compartments. Chloride enters the body alongside sodium.
What is the role of bicarbonate in the body?
Neutralizing pH changes and maintaining acid-base balance in body fluids.
Where is the majority of phosphate in the body found, and what role does it play?
Around 85% of phosphate is located in bone tissue, playing a structural role
How is sulfate primarily generated in the body, and what is its significance?
Sulfate is generated during protein synthesis, contributing sulfur atoms to specific amino acids.
Which electrolytes primarily exist in solid form but also play crucial roles in body fluids?
Calcium and phosphate.
What does pH measure in a solution?
The concentration of hydrogen ions
How does a higher concentration of hydrogen ions affect pH value?
Corresponds to a lower pH value
What can changes in pH due to metabolic reactions significantly affect?
Further metabolic reactions
What is the normal pH range in the blood?
Between 4.35 and 7.45
How do buffers help maintain pH balance in the body?
By absorbing or releasing hydrogen ions to neutralize changes in pH
Which of the following are main mechanisms for maintaining the normal pH range in the body?
Chemical buffers
lungs
kidneys
What is the role of the bicarbonate-carbonic acid buffer system in pH regulation?
Maintaining blood pH around 7.4
How do buffer systems and lungs work together in pH regulation?
To neutralize ongoing hydrogen ion production
Do buffer systems permanently remove hydrogen ions from the body?
No, they neutralize them until they can be exhaled or excreted
How does exhalation contribute to pH regulation?
By expelling carbon dioxide and removing hydrogen ions from the blood.
What effect does high breathing rates have on pH?
High breathing rates raise pH by removing hydrogen ions from the blood.
Where are chemoreceptors located that detect changes in blood pH?
In the medulla oblongata and major arteries.
What happens when blood pH is low?
Low pH can depress the central nervous system, slowing down body functions and inducing drowsiness.
How does breath holding affect blood pH?
Breath holding elevates hydrogen ion levels, leading to a decrease in pH and drowsiness.
What is the primary function of lungs in pH regulation?
Eliminate hydrogen ions by expelling them as water vapor during exhalation.
How do buffer systems contribute to pH regulation?
Absorb hydrogen ions but do not eliminate them.
What role do kidneys play in maintaining pH balance?
Excreting hydrogen ions and other acids like ammonia.
What characterizes respiratory acidosis?
Restricted breathing due to central nervous system issues or airway/lung blockages.
What causes metabolic acidosis?
Inability of the kidneys to handle the acid produced during metabolic processes.
What distinguishes metabolic alkalosis?
Excessive acid loss or elevated bicarbonate levels due to specific kidney conditions.
What is respiratory acidosis?
Occurs when the lungs fail to eliminate enough carbon dioxide, leading to a decrease in blood pH.
What are the causes of respiratory acidosis?
Lung diseases such as COPD and respiratory depression due to central nervous system disorders.
What defines respiratory alkalosis?
Results from hyperventilation, causing an increase in blood pH.
What triggers respiratory alkalosis?
Hyperventilation during panic or trauma and central nervous system disorders.
How is metabolic acidosis defined?
Caused by an excess of acids in the body or loss of bicarbonate ions, leading to a decrease in blood pH.
What are the causes of metabolic acidosis?
Kidney disease, diabetes, and drug overdoses.
How is metabolic alkalosis defined?
A rare condition characterized by excessive loss of acids or elevated bicarbonate levels, resulting in an increase in blood pH.
When does metabolic alkalosis typically occur?
It is typically associated with specific forms of kidney damage.