GCSE Chemistry Paper 1 RPs
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Equipment for salts RP
1.0M dilute sulfuric acid
Copper (II) oxide powder
A spatula
A glass rod
A measuring cylinder
100cm3 beaker
250cm3 beaker
Bunsen burner
Tripod
Gauze
Heatproof mat
Filter mat
Filter funnel and paper
A small conical flask
An evaporating basin
A crystillising dish
Salts Method
1. Measure 20 cm3 sulfuric acid into a measuring cylinder and pour it into beaker.
2. Heat the acid gently using a Bunsen burner.
3. Add small amounts of insoluble base in this case copper oxide in excess (until no more reacts thus no more effervescence is produced).
4. Filter using filter paper and funnel the solution to remove the excess copper oxide.
5. Pour the solution into the evaporating basin.
6. Evaporate the solution using a water bath until crystals start to form.
7. Leave the evaporating basin in a cool place for at least 24 hours.
8. Gently pat the crystals dry between two pieces of filter paper.
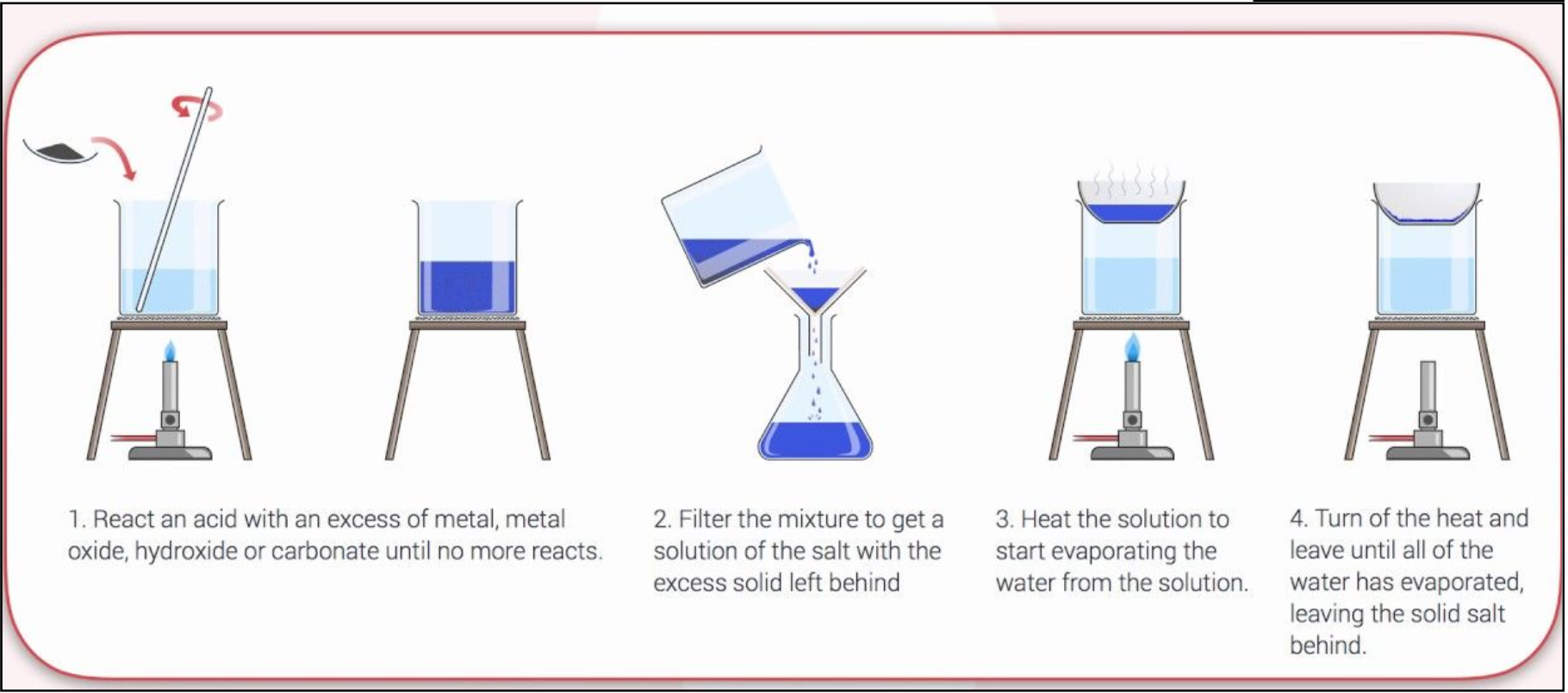
Salts Diagram
React an acid with an excess of metal, metal oxide, hydroxide or carbonate until no more reacts
Filter mix to get a solution of salt with the excess solid left behind
Heat the solution to start evaporating the water from the solution
Turn off the heat and leave until all of the water has evaporated, leaving the solid salt behind.
Safety Precatuions
● Wear safety goggles as sulphuric acid is corrosive.
● Make sure hair is tied back.
● When the Bunsen burner is not in use, turn it off or leave it on the orange safety flame.
Equipment list Neutralisation
● 25 cm3 volumetric pipette
● Pipette filler
● 50 cm3 burette
● 250 cm3 conical flask
● Small funnel
● Clamp stand and clamp
● White tile
● 0.1 M sodium hydroxide solution
● Sulfuric acid
● Phenolphthalein indicator
Neutralisation method
1. Use the pipette to measure 25cm3 of sodium hydroxide into the conical flask.
2. Place the conical flask on a white tile.
3. Fill the burette with sulphuric acid using a funnel.
4. Record the initial reading of acid in the burette. - Make sure to always take readings from the bottom of the meniscus.
5. Add a 5 drops of indicator in this case phenolphthalein to the conical flask.
6. Slowly open the burette tap while swirling the conical flask.
7. Add acid drop-by-drop near the endpoint. - At this point the colour will start to change slightly.
8. Close the burette when a colour change occurs in phenolphthalein. - The solution turns from pink to colourless.
9. Record the final reading of acid in the burette and calculate the titre. This is the volume of acid used to neutralise the alkali.
10. Repeat until you have concordant results.
- These are within 0.1cm of each other.
11. Present results in a table and calculate the mean titre discarding any anomalies when calculating the mean.
12. Calculate the number of moles of sodium hydroxide used in the titration.
13. In the balanced equation the ratio between sodium hydroxide and sulphuric acid is 2:1. Therefore to find out the moles of sulphuric acid divide the moles of sodium hydroxide by 2.
14. Use the formula [concentration= moles/volume (mean titre volume)] to work out the concentration of sulphuric acid.
![<p>1. Use the pipette to measure 25cm3 of sodium hydroxide into the conical flask. </p><p>2. Place the conical flask on a white tile. </p><p>3. Fill the burette with sulphuric acid using a funnel. </p><p>4. Record the initial reading of acid in the burette. - Make sure to always take readings from the bottom of the meniscus. </p><p>5. Add a 5 drops of indicator in this case phenolphthalein to the conical flask. </p><p>6. Slowly open the burette tap while swirling the conical flask. </p><p>7. Add acid drop-by-drop near the endpoint. - At this point the colour will start to change slightly. </p><p>8. Close the burette when a colour change occurs in phenolphthalein. - The solution turns from pink to colourless. </p><p>9. Record the final reading of acid in the burette and calculate the titre. This is the volume of acid used to neutralise the alkali.</p><p>10. Repeat until you have concordant results. </p><p>- These are within 0.1cm of each other. </p><p>11. Present results in a table and calculate the mean titre discarding any anomalies when calculating the mean. </p><p>12. Calculate the number of moles of sodium hydroxide used in the titration. </p><p>13. In the balanced equation the ratio between sodium hydroxide and sulphuric acid is 2:1. Therefore to find out the moles of sulphuric acid divide the moles of sodium hydroxide by 2. </p><p>14. Use the formula [concentration= moles/volume (mean titre volume)] to work out the concentration of sulphuric acid.</p>](https://knowt-user-attachments.s3.amazonaws.com/40e7ea29-6d4e-42ec-b82f-1e384380e058.png)
Neutralisation RP Safety Precautions
● Wear safety goggles whean working with acids.
● Tie hair back.
● Report any broken glassware immediately.
Neutralisation Aim
Carry out an investigation to find the concentration of a dilute sulfuric acid solution, using a sodium hydroxide solution of known concentration.
H2SO4 + 2NaOH ----> Na2SO4+2H2O
Aim of Temperature Changes RP
Investigate the variables that affect temperature change in chemical reaction
Equipment Temperature RP
● 2 M hydrochloric acid
● 2 M sodium hydroxide solution
● Expanded polystyrene cups and lids with thermometer holes
● Thermometers
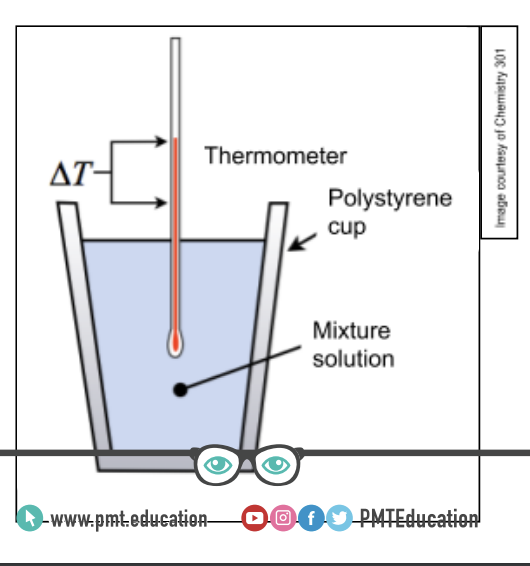
Method TEMP RP
1. Measure 25cm3 of hydrochloric acid into a polystyrene cup.
2. Place the cup inside the beaker to make it more stable.
3. Measure and record the temperature of the hydrochloric acid.
4. Measure 5cm3 of sodium hydroxide and add it to the polystyrene cup.
5. Quickly put a lid on the cup and gently stir the solution with the thermometer through the hole of lid.
6. When the reading on the thermometer stops changing and becomes fairly constant, record the temperature.
7. Repeat steps 4 and 5 to add further 5 cm3 amounts of sodium hydroxide to the cup. A minimum total of 40 cm3 needs to be added
. 8. Repeat steps 1–7 to ensure reliability of results.
9. Calculate the mean maximum temperature reached for each of the sodium hydroxide volumes
Safety Precautions Temp RP
Wear safety goggles
Aim is Electrolysis RP
Investigate what happens when aqueous solutions are electrolysed using inert electrodes.
Electrolysis RP Equipment
● 0.5 M copper(II) chloride solution
● 0.5 M sodium chloride solution
● A petri dish lid with bored holes
● Two carbon rod electrodes with support bungs
● Two crocodile/4mm plug leads
● Low voltage power supply
● Blue litmus paper
● Forceps
Electrolysis RP method
1. Add about 50cm3 of copper chloride solution to a beaker.
2. Add the lid and insert electrodes through the holes making sure the electrodes don’t touch.
3. Attach crocodile leads to the electrode and connect the rods to the DC terminals of a low voltage power supply. 4. Set the power supply to 4V and switch the power supply on. 5. Using the forceps hold the litmus paper near the positive electrode. 6. After a few minutes turn the power supply off and observe the negative electrode. 7. Record observations at the electrodes.
1. Add about 50cm3 of sodium chloride solution to a beaker.
2. Add the lid and insert electrodes through the holes, making sure the electrodes don’t touch.
3. Attach crocodile leads to the electrode and connect the rods to the DC terminals of a low voltage power supply.
4. Set the power supply to 4V and switch the power supply on.
5. Using the forceps hold the litmus paper near the positive electrode.
6. After a few minutes turn the power supply off and observe the negative electrode. There should be effervescence.
7. Record observations at the electrodes.
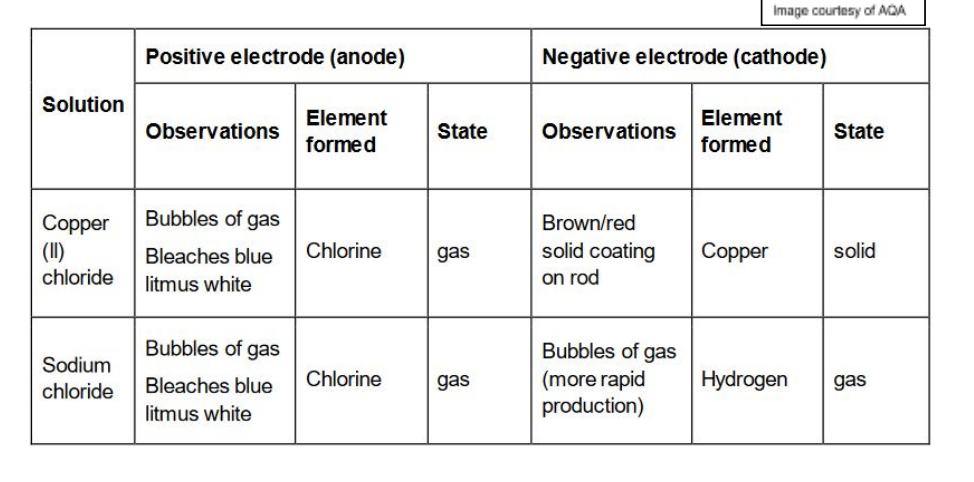
Results of Electrolysis RP
Copper (II) Chloride and Sodium Chloride at positive electrode : Bubbles of gas, Bleaches blue litmus white, Chlorine formed, Gas
Copper (II) Chloride at negative electrode : Brown/red solid coating on rod, Copper formed, Solid
Sodium Chloride at negative electrode: Bubbles of gas (more rapid production), hydrogen formed, gas
Safety precautions of electrolysis RP
Safety goggles must be worn.
Room should be well ventilated because large quantities of chlorine gas is toxic.