General Chemistry Final Exam
1/201
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
202 Terms
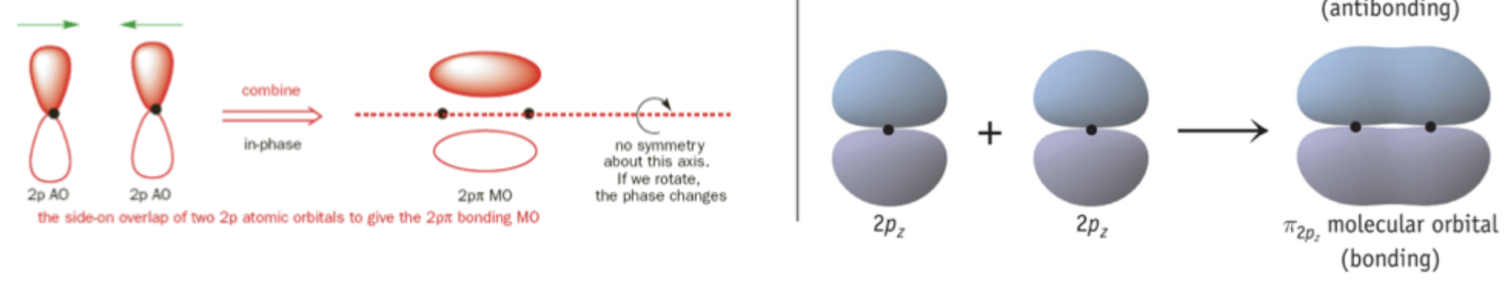
Pi bonds are formed from
unhybridized p orbitals
Pi bonds occur due to a ________ interaction
side on
Valence Bond Theory vs. Molecular Orbital Theory
Valence Bond Theory:
Both depend on geometry and shape
Electrons are localized in a given bond/lone pair
Bond is overlap of half filled atomic orbitals
Molecular Orbital Theory
Both depend on geometry and shape
Electrons are delocalized across the whole molecule (Resonance Structures)
Bonding is a mix of atomic orbitals on all atoms, and molecular orbital is spread over multiple atoms
Lewis Structures fail to explain which concept?
Diamagnetic vs Paramagnetic (example of O2 which appears to be diamagnetic, but it is actually paramagnetic)
Diamagnetic
All electrons are paired
Paramagnetic
At least one electron is unpaired
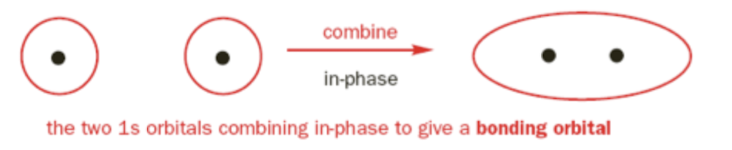
Constructive Interference
occurs when two atomic orbitals of the same phase meet each other. the electron cloud (molecular orbital) interacts with both nuclei and is more stable.
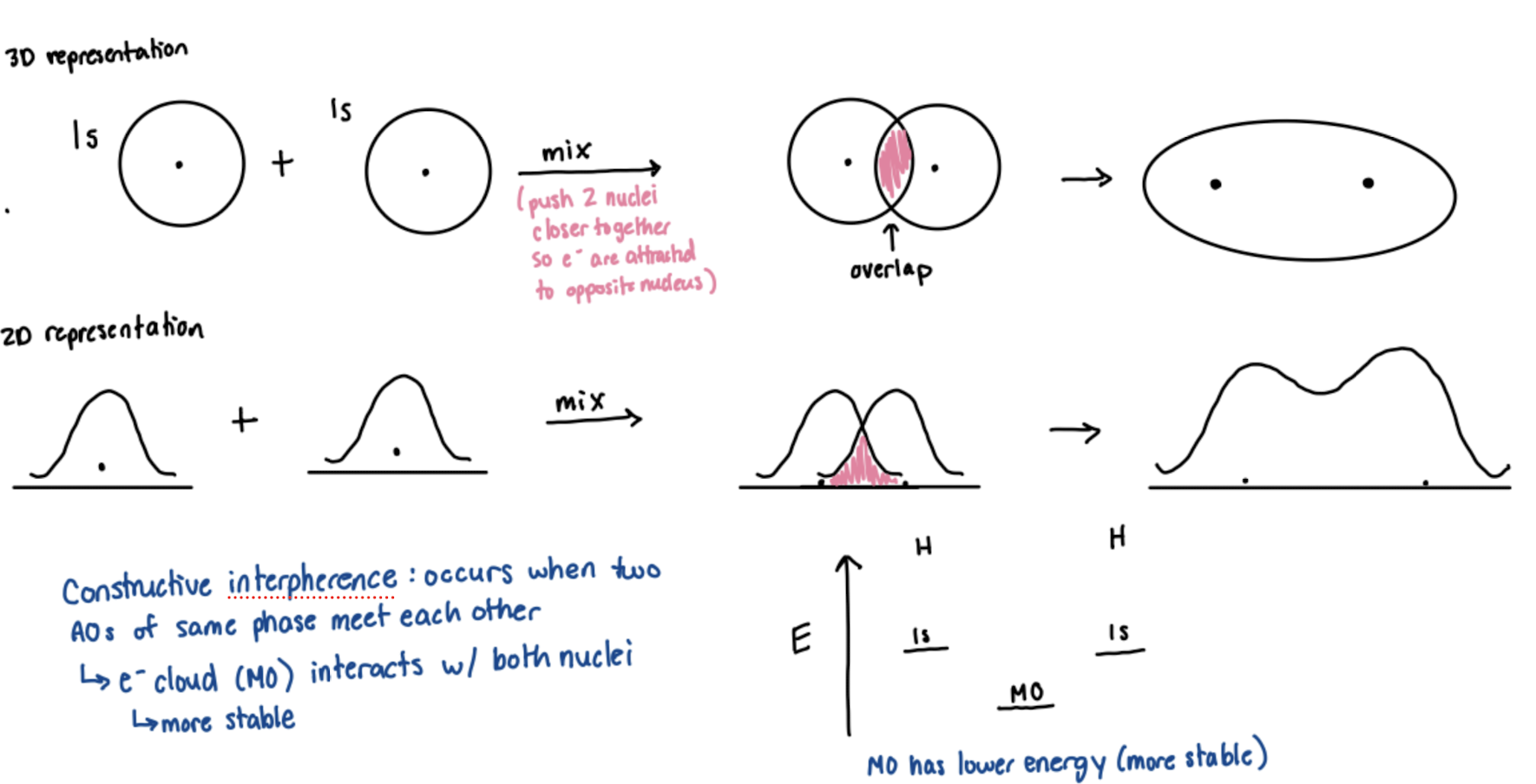
What bonding character occurs with constructive interference?
Bonding Molecular Orbital
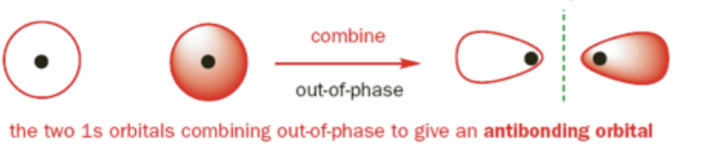
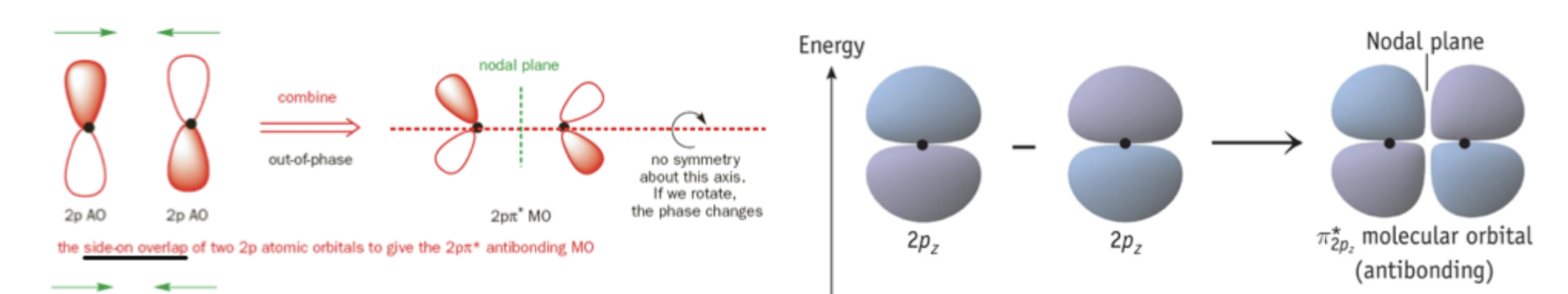
Destructive Interference
occurs when two atomic orbitals of opposite phase meet each other. electron cloud (molecular orbital) interacts less with each nuclei, a node is seen, and it is less stable.
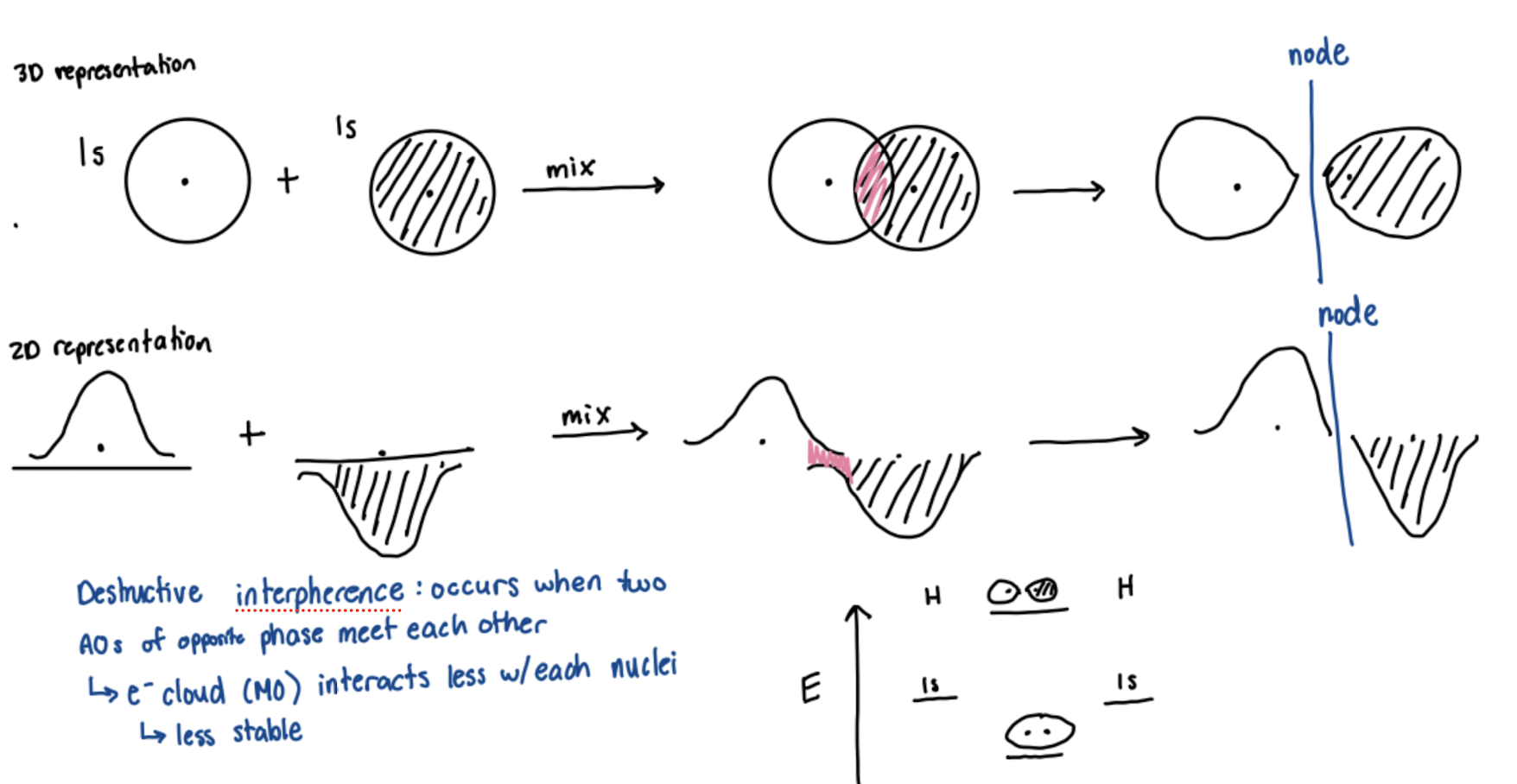
What bonding character occurs with destructive interference?
Antibonding molecular orbital
Bonding orbitals are ____ stable than antibonding orbitals
more stable, lower in energy
Correlation Diagrams
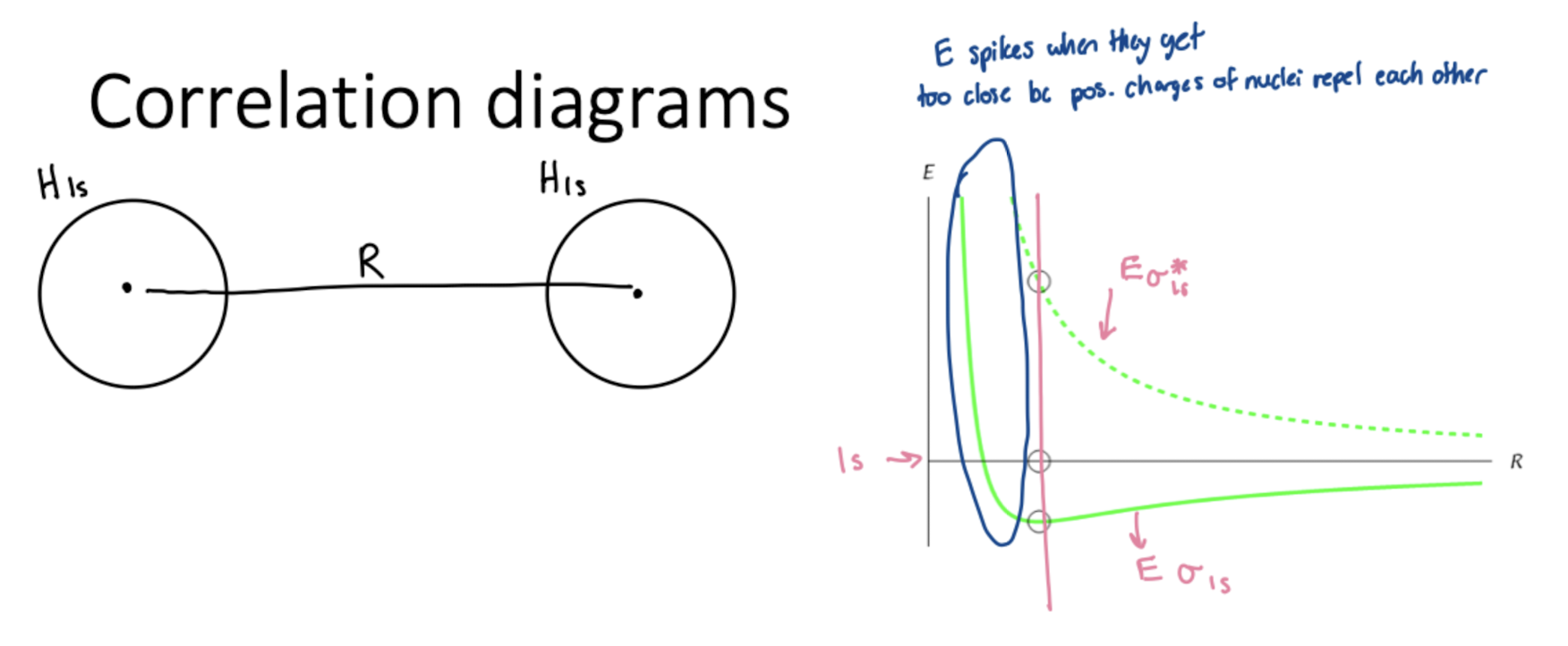
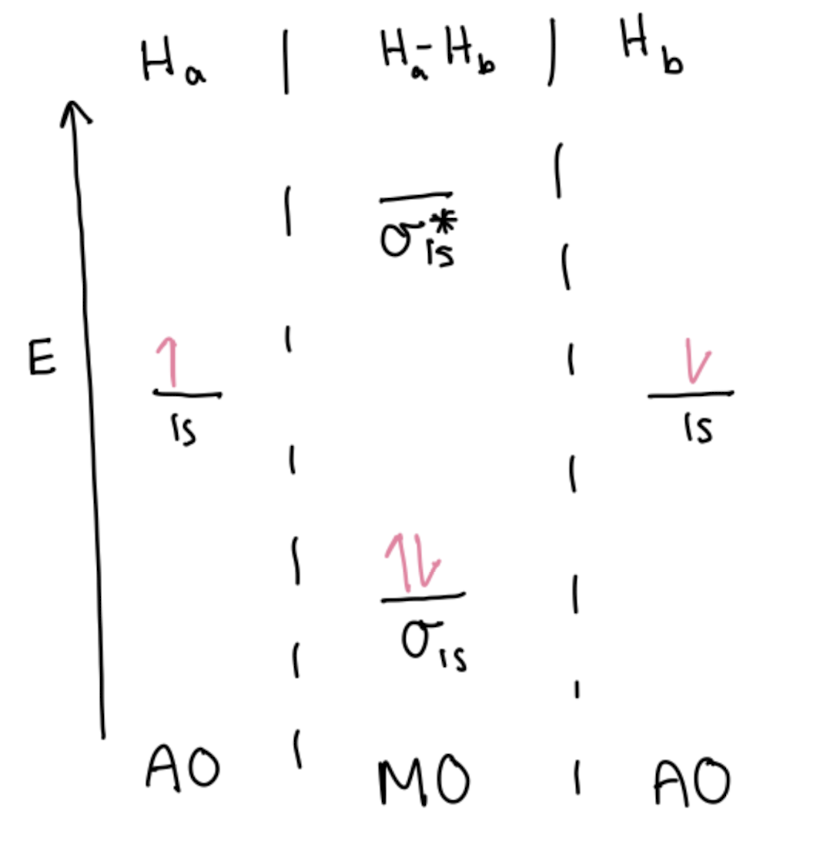
Bond Order
(Number of bonding e - number of antibonding e) / 2
As bond order increase, bond length…
decreases
As bond order increases, bond strength…
increases
What does a bond order of zero mean?
Means that the molecule cannot form. Noble gases are example of this because they have bond order zero, and prefer to exist with an octet of lone pairs instead of forming bonds
Why can core electrons be left out of correlation diagrams?
They do not contribute to bonding
Core electrons do not overlap well
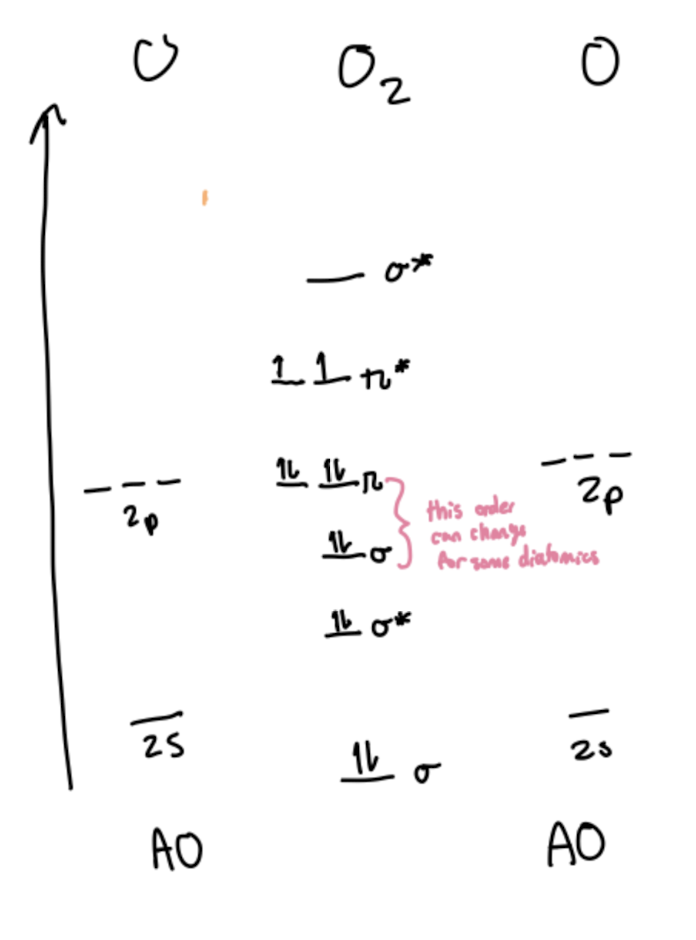
σ1s
σ*1s
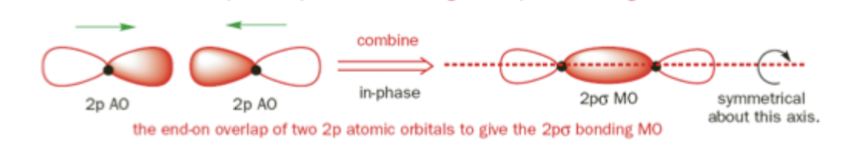
σ2pz
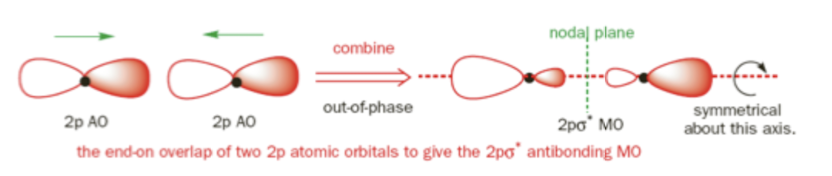
σ*2pz
π2px or y
π*2px or y
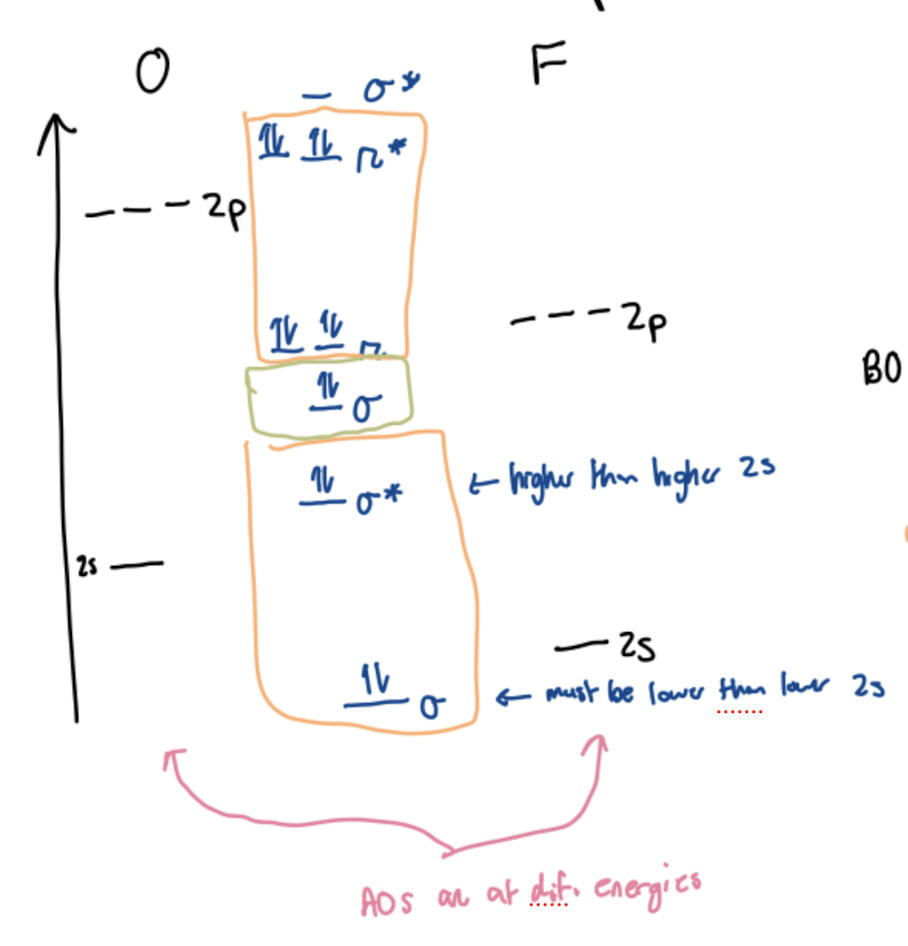
Molecular Orbital Diagram for O2
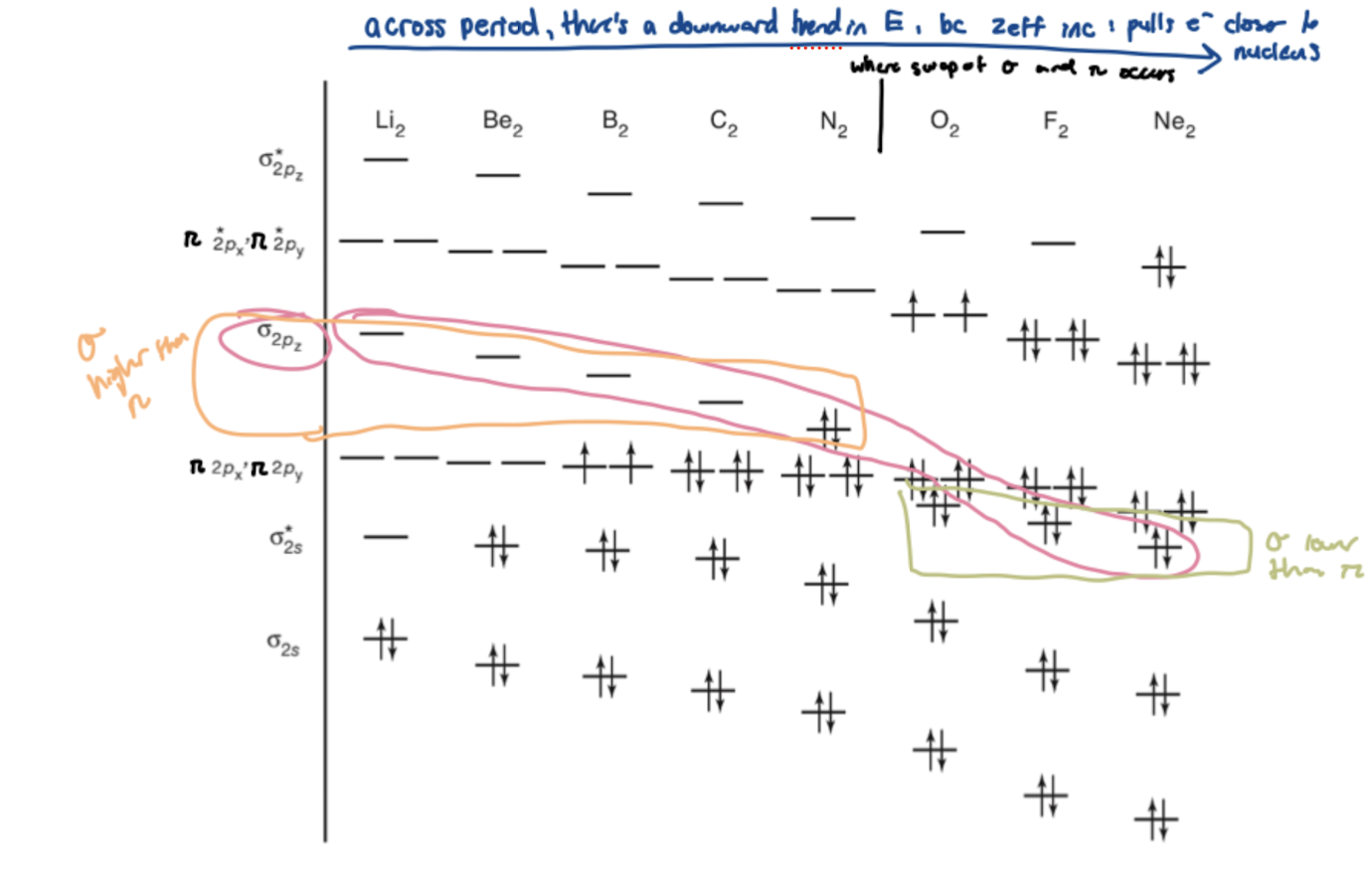
Homonuclear diatomics ordering
For diatomics N and earlier, σ2pz is higher in energy than π2px and y
For diatomics O and later, σ2pz is lower in energy than π2px and y
This is due to the downward trend in Ionization Energy 1 as your move across a period (since zeff increases and electrons are pulled closer to nucleus)
Polar bonds in heteronuclear diatomics are formed from…
electrons that do not cancel each other out (do not have a bonding/antibonding cancellation) SHOWN IN GREEN
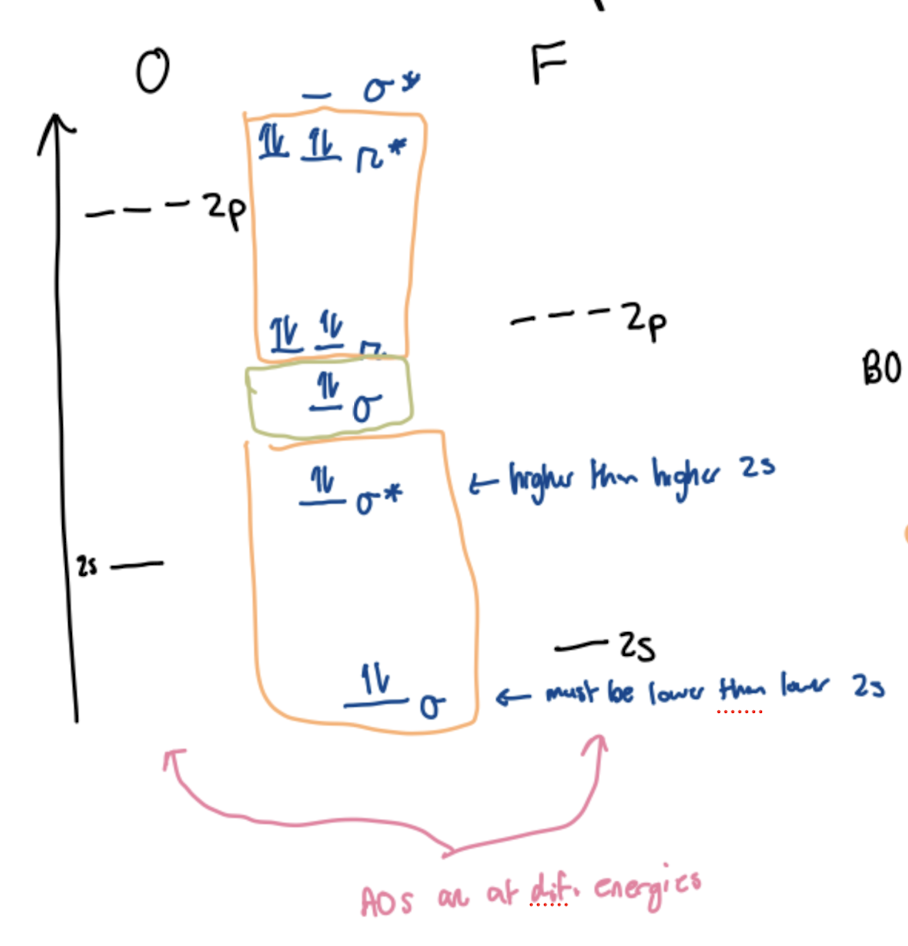
Lone pairs in heteronuclear diatomics are formed from…
electrons that get cancelled between bonding and antibonding orbitals SHOWN IN ORANGE
What attracts a molecule to another molecule?
Ion-Ion
Intermolecular Forces
What to consider when determining molecular polarity?
Lewis Structure
Shape (Molecular Geometry)
Bond Dipoles (Electronegativity differences)
Do Bond Dipoles Cancel? (only if symmetric shape and all bonded atoms on outside are the same)
If cancel, non-polar
If do not cancel, molecule is polar
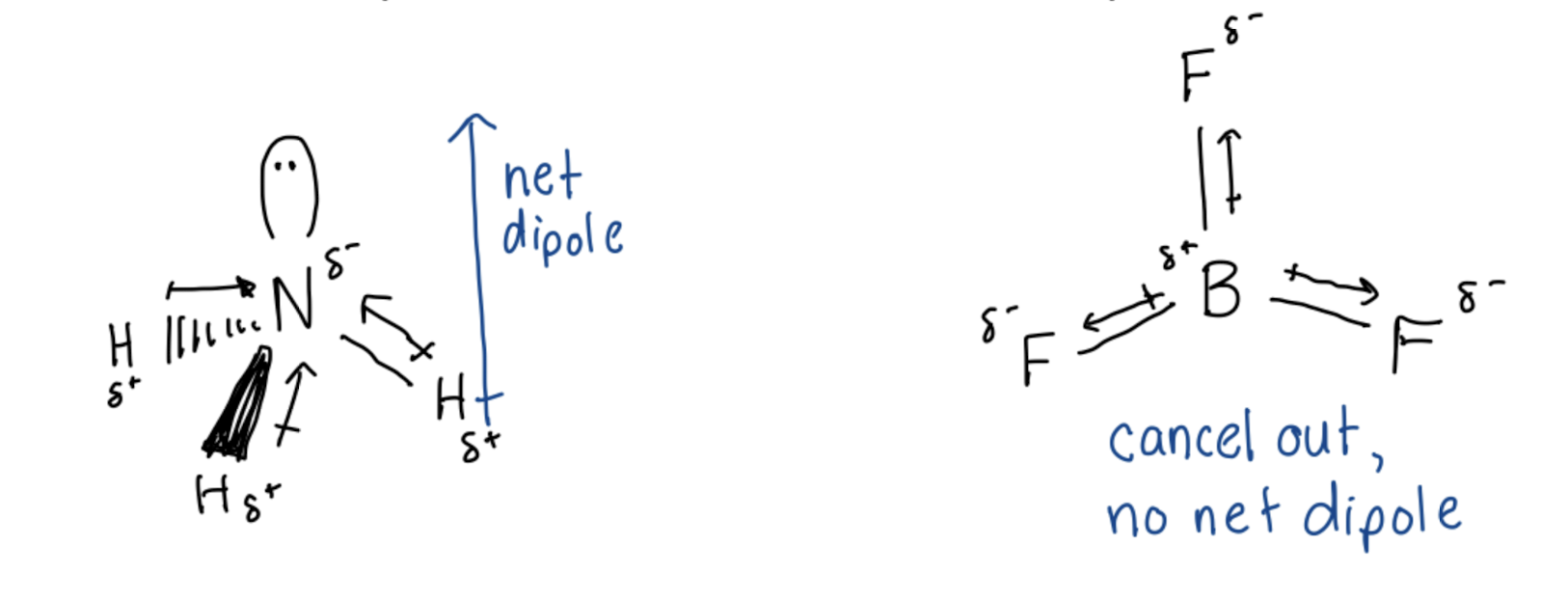
Types of Intermolecular Forces
Dipole Dipole
Hydrogen Bonding
Dispersion Forces
Dipole Dipole
Interactions between partial charges on polar molecules. Can be attractive (opposite poles) repulsive (same pole) or no impact (one molecule upright and one sideways)
Hydrogen Bonding
H bonding is a particularly strong dipole-dipole interaction between electron poor H (bound to O, N, or F) with a particularly small electron rich element (O, N, or F) with a lone pair
Dispersion Forces
weak attractive forces between atoms and molecules caused by temporary induced dipoles. Always attractive regardless of orientation
Stronger dispersion forces are found in _______
Molecules with bigger electron clouds
Why is ice less dense than liquid water?
The lattice-like structure that forms as a result of hydrogen bonding in solid water forces molecules to be further apart, which means it is less dense than liquid water
Ice: 2 h-bonds per water molecule
Liquid water: 1.8 H-bonds per water moleule
As strength of IMFs increases, boiling point ______.
increases
As strength of IMFs increases, surface tension ______.
increases
As strength of IMFs increases, viscosity ______.
increases. Inversely proportional to temp
As strength of IMFs increases, heat of vaporization ____.
increases
Comparing strength of IMFs
Always compare molecules of similar size (similar number of large, non H atoms)
Then, H bonds > Dipole-Dipole > Dispersion (since force is the same in both molecules)
Evaporation
molecules on the surface with sufficient energy break IMFs. Needs no input of outside energy (always happening in a sample at some rate)
Rate of Evaporation
Constant at a given temperature
Increases with increasing temperature (avg. kinetic energy increases)
Condensation
If a gas particle hits the surface with low enough energy, it will not have enough energy to move away due to IMFS
rate increases with increased SA
rate increases with increased IMFs
Vapor Pressure
Gas pressure when the rate of evaporation = rate of condensation; where PH20,vap=PH20, liquid Increases as temp increases
Lower vapor pressure means _____ IMFs
stronger (because rate of condensation is higher with stronger IMFs meaning rate evap = rate condensation faster
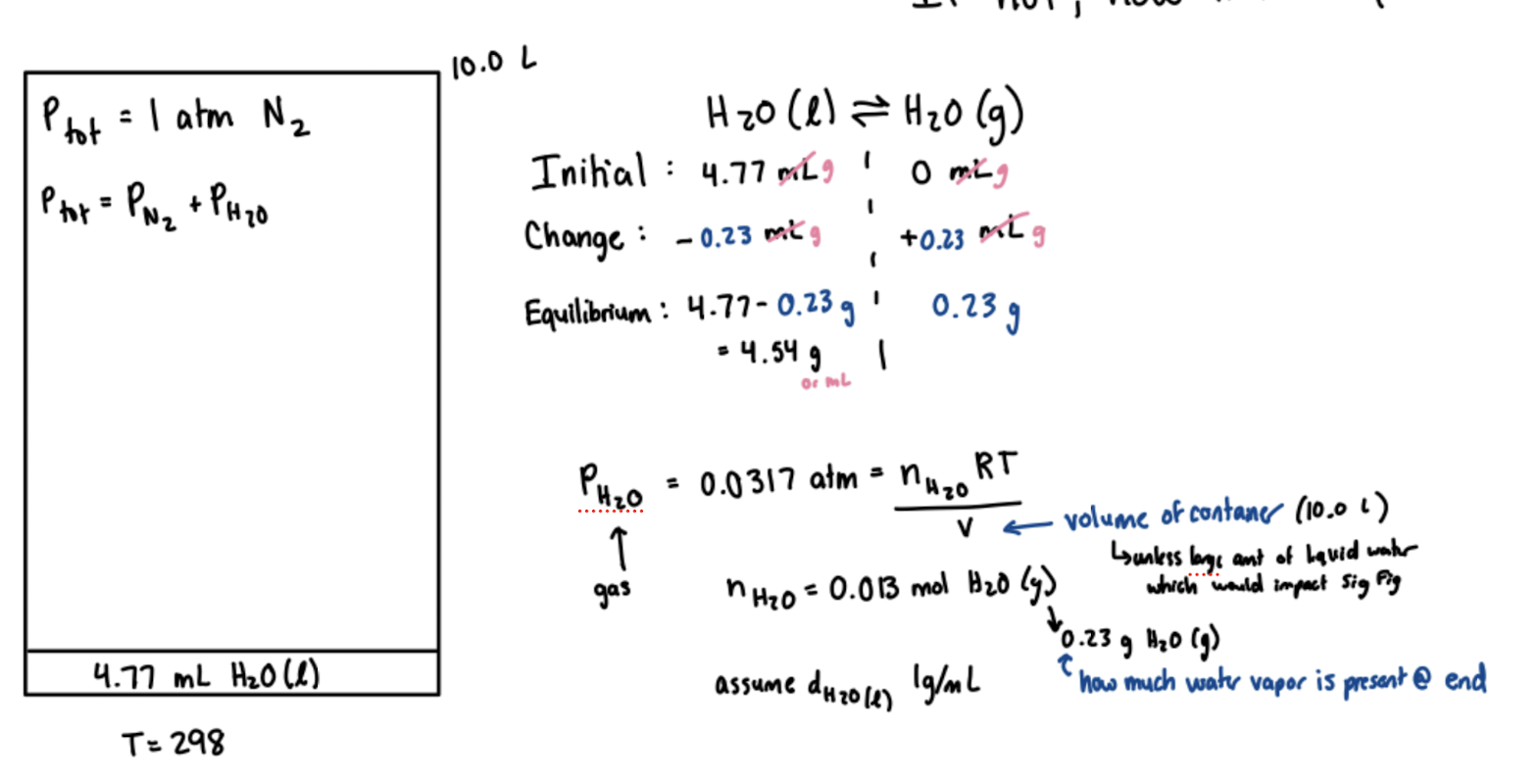
What occurs in a sealed container
At Start
Total pressure = 0 (no gas)
Rate of evaporation > Rate of Condensation (Because no gas currently)
As Time Passes:
H20 liquid decrease
H20 gas increase
Rate of evaporation stays the same
Rate of condensation increases as PH20 increases over time
Eventually
Reach the vapor pressure of water for whatever temp this happens at
Rate evaporation = rate condensation
Boiling Point
Temperature at which vapor pressure=external pressure
If pressure increases, the boiling point increases
If given temperature and vapor pressure for a sealed container, can determine how much water will evaporate….
Vapor pressure = Pressure H20 (g)
Set up PV=nRT using vapor pressure for P and volume of container for V to find moles of H20 gas at the end
Use moles H20 gas to find grams H20 gas (then if its water, density of 1 g/mL means this amt can apply for mL or grams), then subtract this from initial water mass and add to initial mass of gas
Boiling
occurs throughout when Patm= Pvap
Boiling point is higher when pressure is higher
Use mobjCsΔT when…
finding q for warming/cooling, ΔKE
Use nrxnΔHrxn when…
finding q for a phase change
ΔHfusion compared to ΔHvaporization
ΔHfusion < ΔHvaporization because for fusion to occur, only need to break some IMFs vs vaporization where all IMFs must be broken
Solid to liquid
ΔHfusion Endothermic. Can also be known as melting
Liquid to gas
ΔHvaporization Endothermic. Can also be known as boiling.
Gas to liquid
-ΔHvaporization Exothermic. Can also be known as condensation
Liquid to solid
-ΔHfusion Exothermic. Can also be known as freezing
Solid to gas
Sublimation
Gas to Solid
Deposition
Showing change in temperature with phase change (heating curve)
Must get substance to freezing/boiling point using mobjCsΔT, then use nrxnΔHrxn for the phase change at that temperature. Slopes represent heating/cooling and horizontal lines represent phase changes. Shown at constant temp
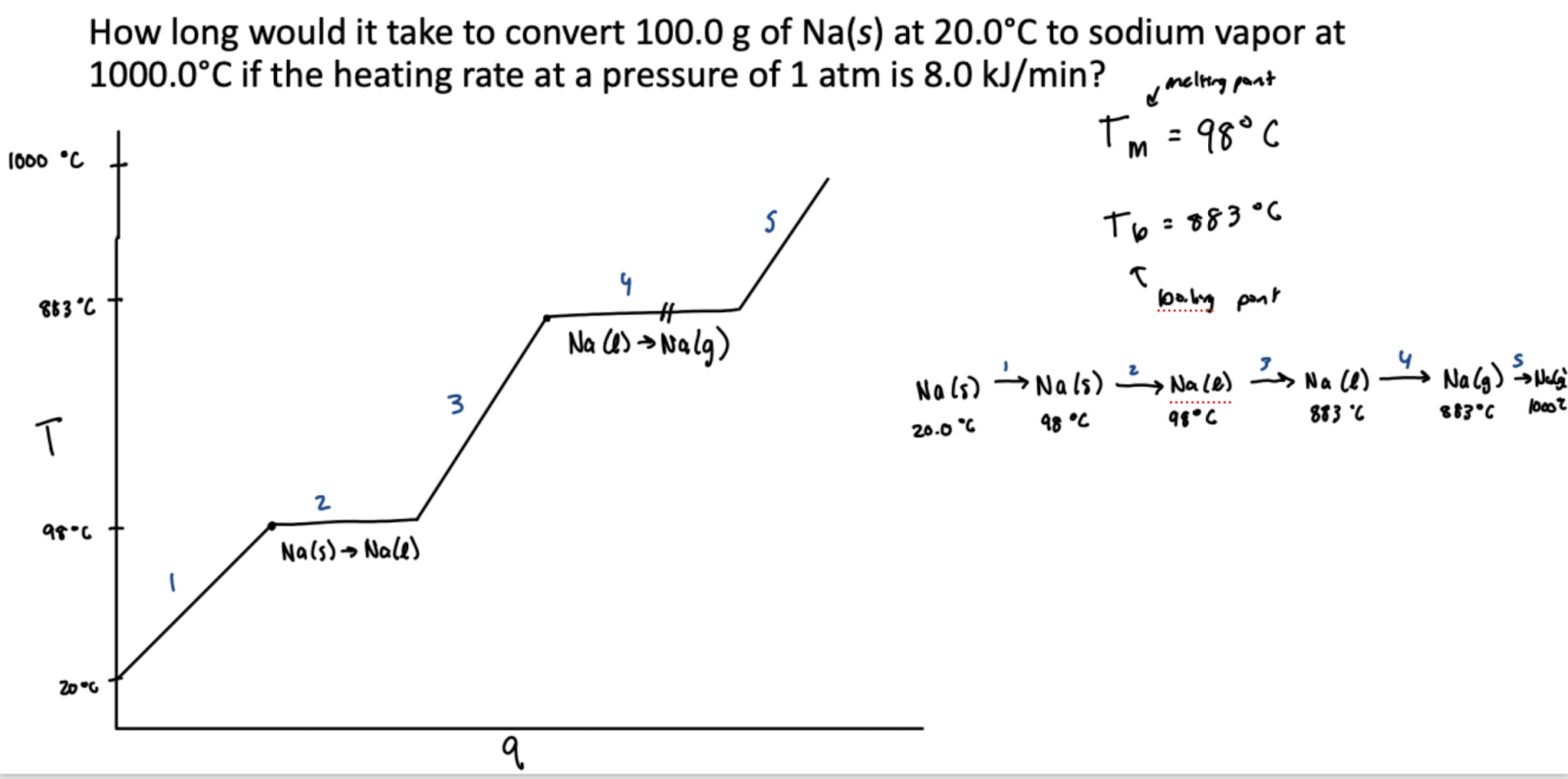
Phase diagrams
Show phases at different temperatures and pressures (on left).
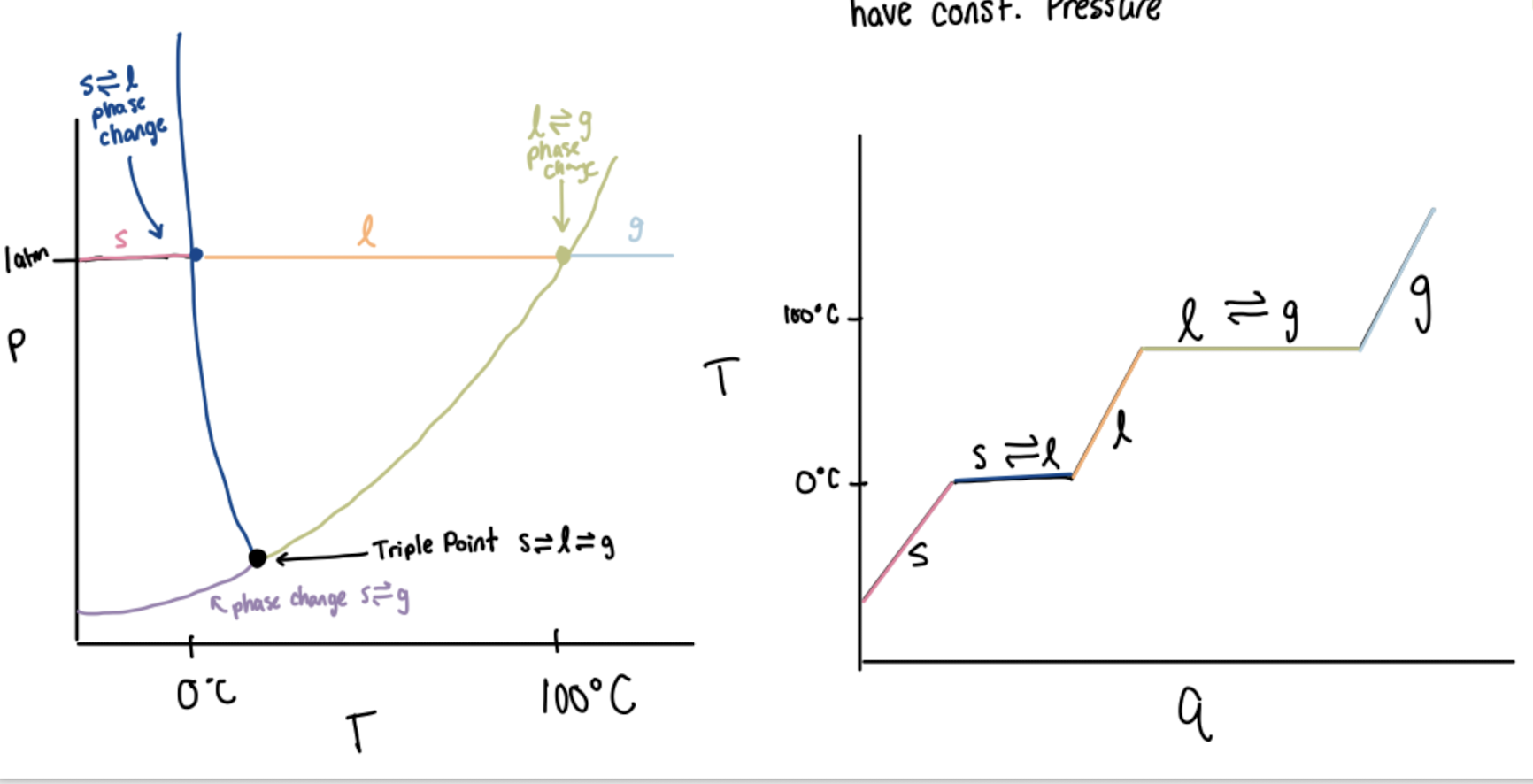
Triple Point
Temperature and pressure (shown by all lines intersecting) where all phases are present
Critical Point
Above this point, you have a supercritical fluid
Supercritical fluid
density similar to liquid
viscosity similar to gas
fills container like a gas
If solvent-solute interactions > solvent-solvent and solute-solute interactions
ΔHsoln< 0 (exothermic)
Solution forms
If solvent-solute interactions = solvent-solvent and solute-solute interactions
ΔHsoln = 0
Solution forms
If solvent-solute interactions < solvent-solvent and solute-solute interactions
ΔHsoln > 0 (endothermic)
Solution may form
ΔHsoln=
break solvent-solvent interactions (endo) + break solute-solute interactions (endo) + form solute-solvent interactions (exo)
DRIVING FORCE IS ENTROPY
Entropy
measurement of matter or energy dispersal in a system
more arrangements, more entropy
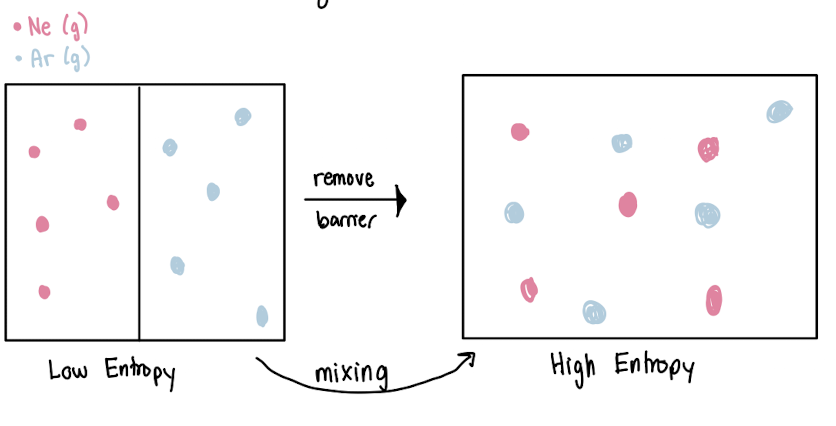
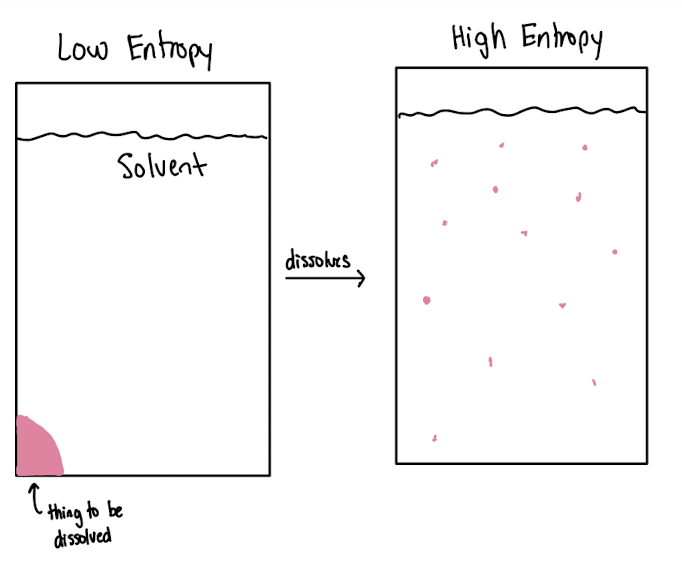
solubility
maximum amount of solute able to be dissolved in a solvent under given conditions
solubility = max amt of solute / amt solvent
unsaturated solution
concentration < solubility
rate dissolution > rate of recrystallization
saturated solution
concentration = solubility
rate dissolution = rate of recrystallization
oversaturated solution
concentration > solubility
rate dissolution < rate recrystallization
miscible
mix in any ratio (no solubility limit)
solubility: gases with gases
always miscible
solubility: liquids with liquids
like dissolves like (in terms of polarity)
concentration
amt solute / amt solution
molarity: mol/L
mass %: mass/mass x 100%
mole fraction: moles/total moles
Energy diagrams for solution formation
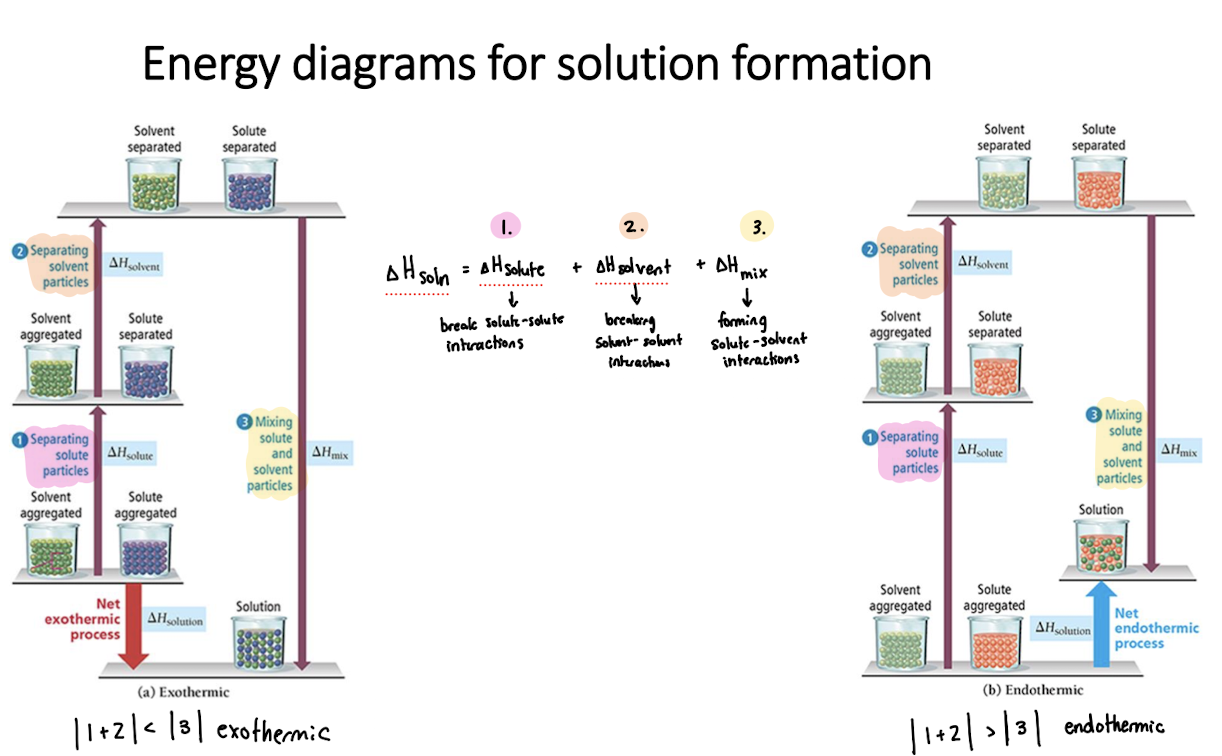
Dissolution of Ionic and Covalent Compounds
Ionic: dissociate into individual ions which are surrounded by solvent
Covalent: dissolve as whole molecules (not breaking covalent bond)
electrolyte
ions in solution conduct electricity (produce ions when put in water)
ionic species and dissolves —> strong electrolyte
strong electrolyte
100% of products form
weak electrolyte
< 100% of products form
non-electrolyte
no products form (aka pathetic)
some molecular solutes form ions by reacting
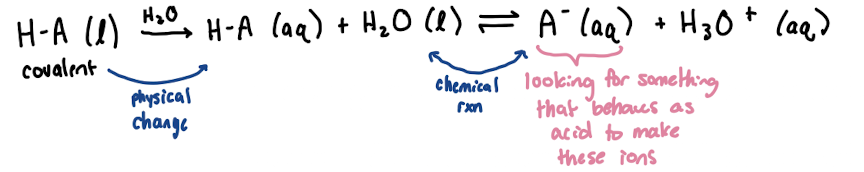
Solution equilibrium
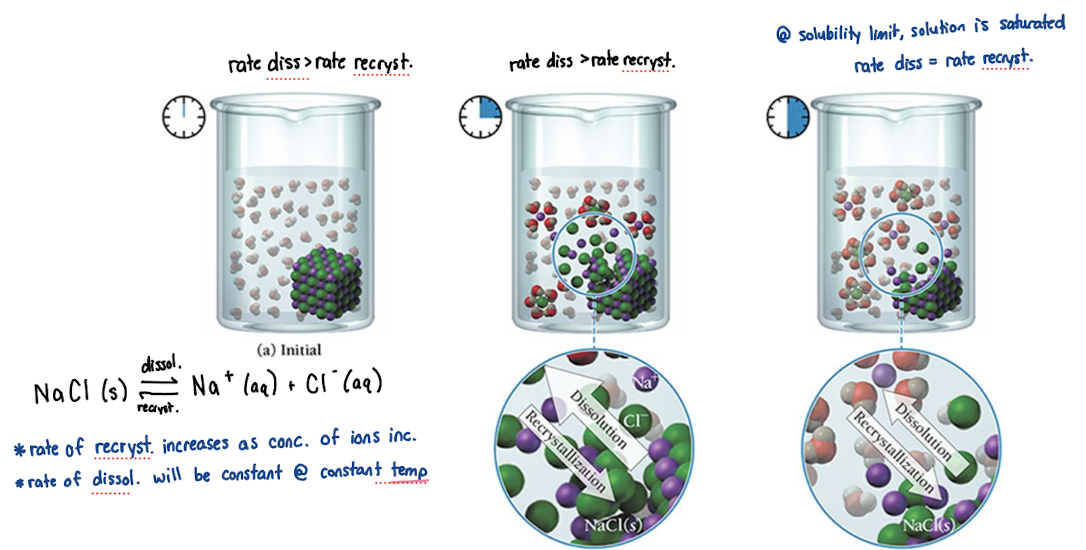
solubility curves for solids
solubility of a substance v. temperature
With Increased Temperature:
increased rate of dissolution (by a lot)
increased rate of recrystallization (by a smaller factor)
so, solubility typically increases for solids at higher temperatures
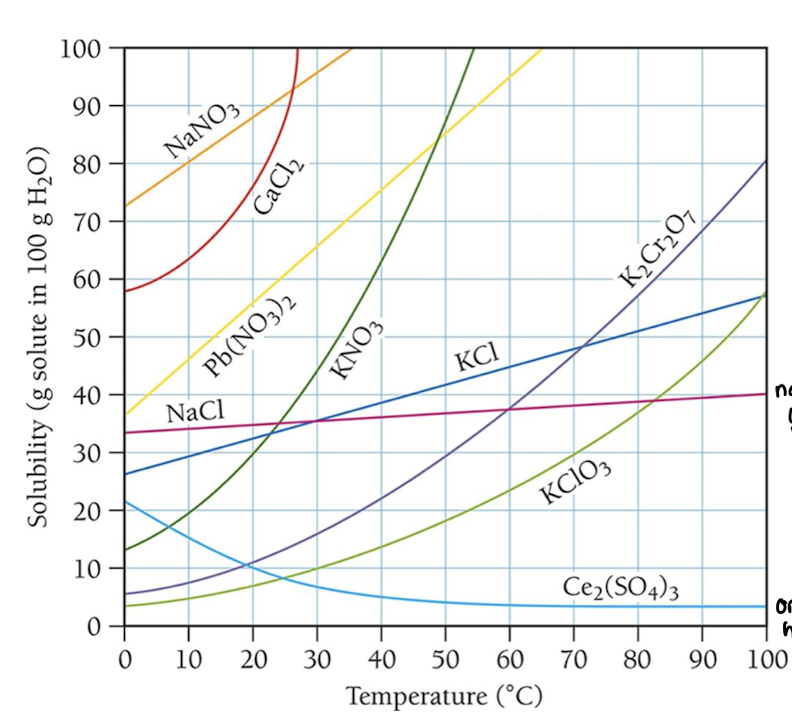
temperature dependence of solubility of gases in water
increase of temperature, decrease of solubility
With Increased Temperature:
increased rate of dissolution (by a smaller factor)
increased rate of gas bubbling out (by a lot!)
so, solubility typically decreases for gases at higher temperatures
impact of temperature on solubility of solids v. gas
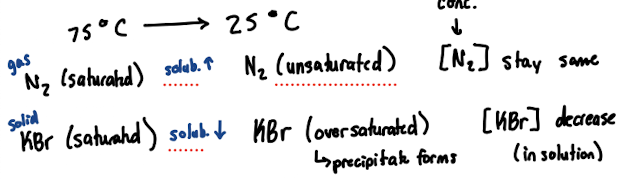
pressure dependence of solubility of gases in water
(refers to the partial pressure of that gas above water: not dependent on pressures of other gases)
as pressure of that gas increases, solubility increases (more will end up dissolves in the water)
Henry’s Law
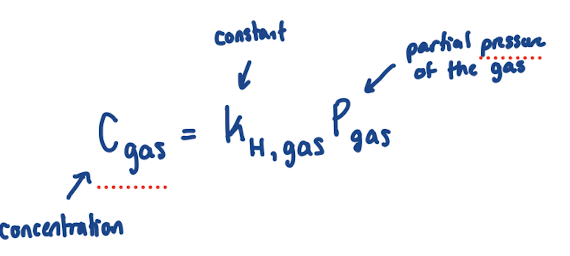
Concentration: molarity of gas in aqueous solution
Pgas: pressure of gas above liquid
molarity (M)
moles solute / L solution
molality (m)
moles solute / kg solvent
mole fraction (x)
moles solute / moles solution
(then multiply this by 100% for mole percent)
colligative properties
Nothing to do with the identity of the solute, only dependent on the amount
Boiling Point Tb
Freezing Point Tf
Vapor Pressure (Pvap)
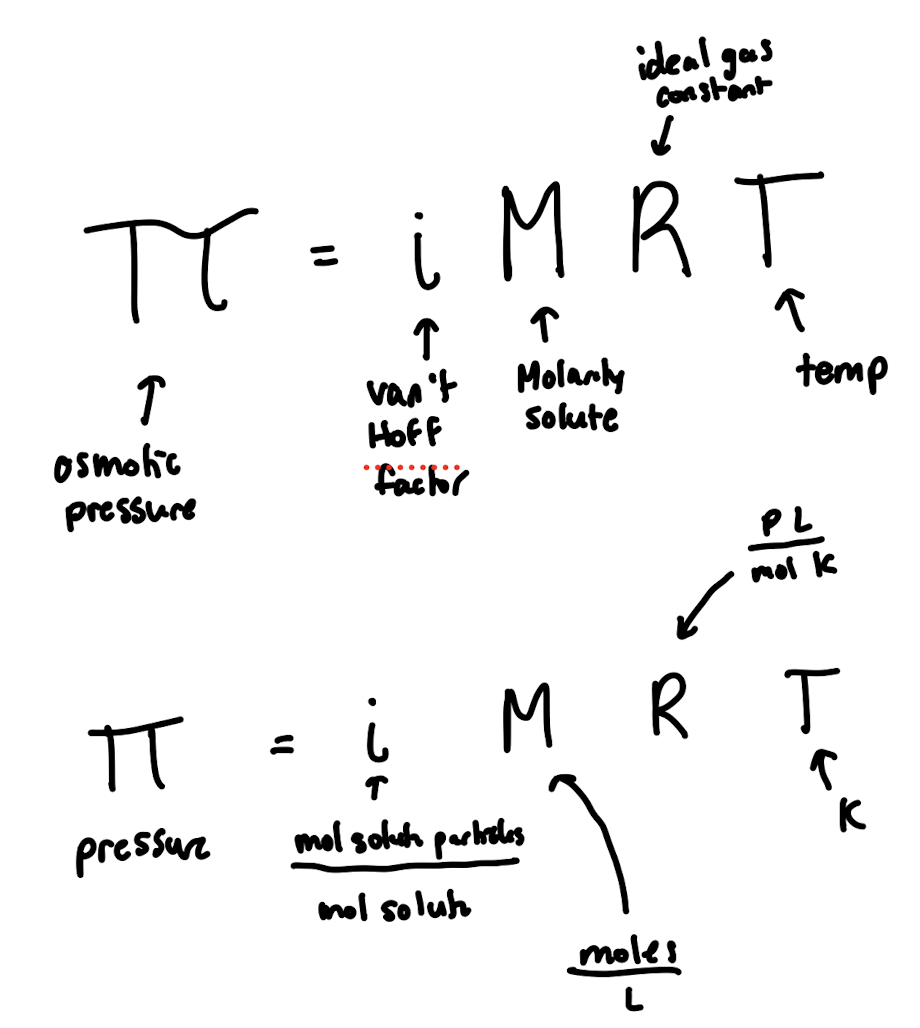
Osmotic Pressure
Freezing Point Depression: ΔTf=
i x m x kf
i: Van’t Hoff Coefficient (for electrolytes), moles of particles formed in solution / moles solute added
m: molality of particles
kf : freezing point constant (unique to the solvent)
Boiling Point Elevation: ΔTb=
i x m x kb
i: Van’t Hoff Coefficient (for electrolytes), moles of particles formed in solution / moles solute added
m: molality of particles
kb : boiling point constant (unique to the solvent)
Equation for vapor pressure after dissolution of a compound (when solutes are nonvolatile)
Pvap, soln = xsolvP°vap,solv
xsolv: mole fraction (aka how much of the surface is still solvent particles)
P°vap,solv : vapor pressure of pure solvent
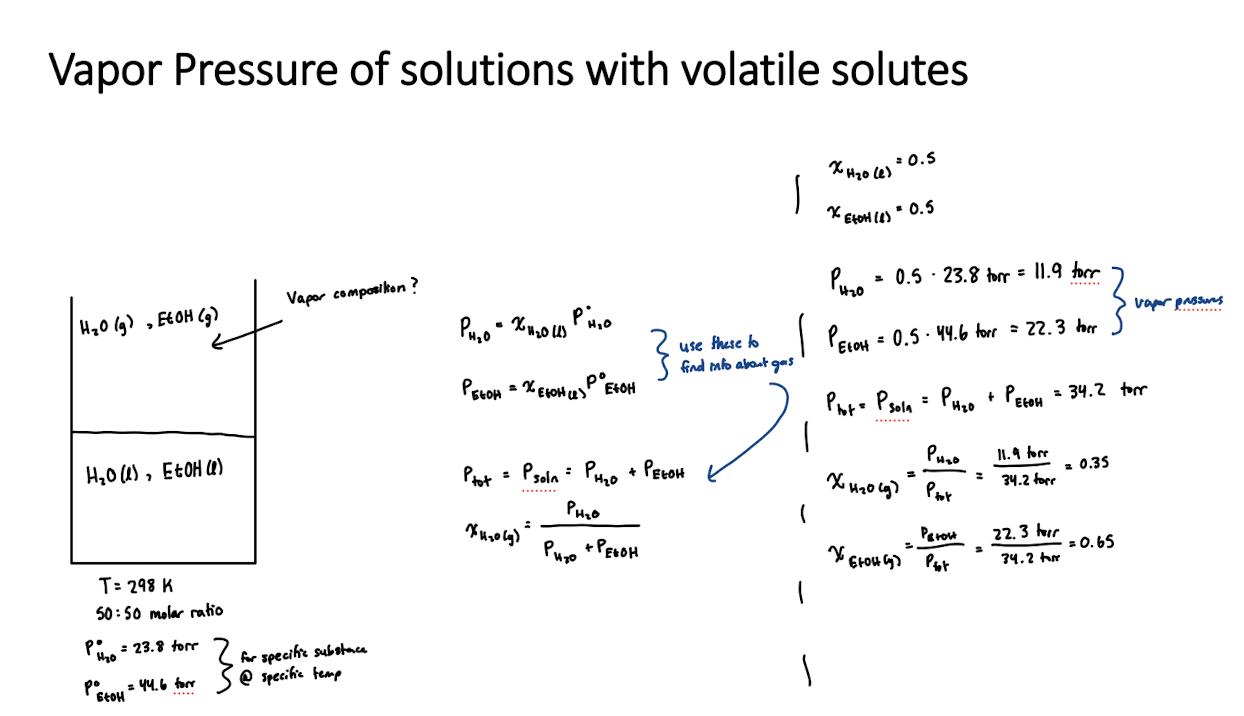
Equation for vapor pressure after dissolution of a compound (when solutes are volatile)
This means that evaporation of solute must be accounted for!
Pvap, soln = xAP°vap,A + xBP°vap,B
same as Ptot = PA + PB
Osmotic Pressure
Important Equations to Consider with Ch. 11 Questions
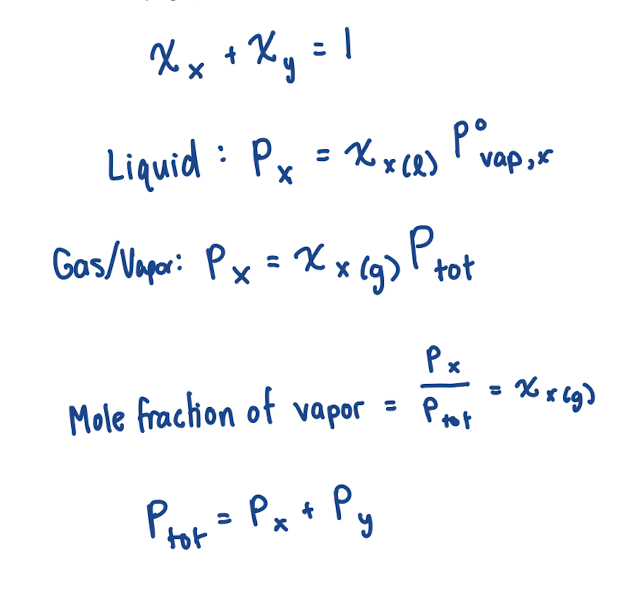
Equation for average rate of consumption or production of a substance, A
Δ[A] / Δt