molecular energy storage and particle in a box
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
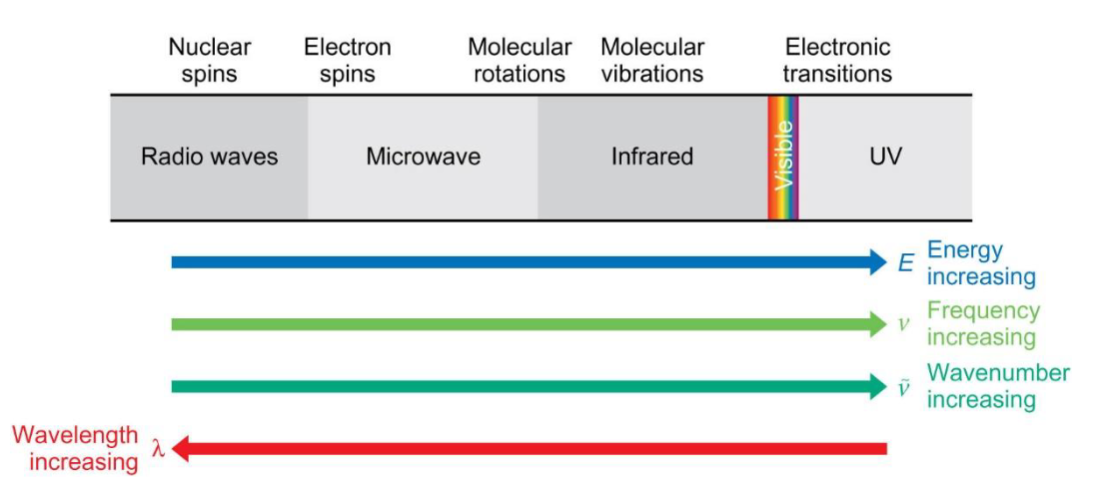
em spectrum (no x-ray or gamma) from lower to higher energy
assign: molecular vibrations, nuclear spins, electronic transitions, molecular rotations and electron spins to which region
label: increasing E, increasing frequency, increasing wavenumber and increasing wavelength
how are different forms of energy storage treated and why (give an equation)
various forms of energy in a molecule can be treated differently
this is because they occur on different time scales
these energies are quantised and most (except translational) can be measured by spectroscopy
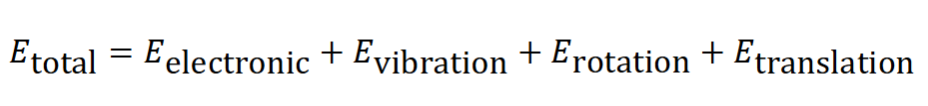
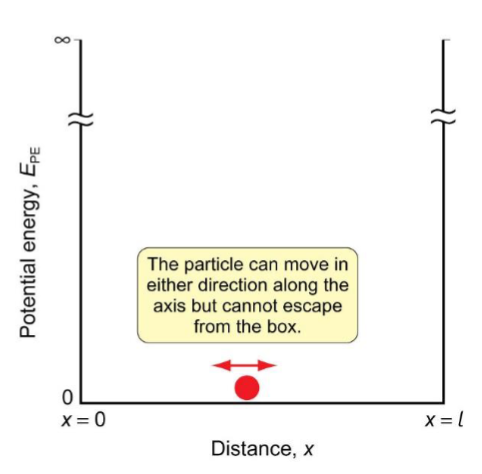
x and V for a particle confined to a 1D box of length l
diagram - “y” axis, “x” axis, movement of particle
inside: 0 ≤ x ≤ l and V(x) = 0
outside: x ≤ 0 or x ≥ l. V(x) = ∞ → the particle cannot exist in these regions of space where ψ (x) = 0
from previous set: what is the solution (for wavefunction) to the schrodinger equation in regions of constant potential?
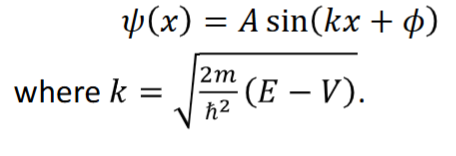
boundary conditions for particle in a box and why these are defined
what does this mean for allowed energies
as ψ (x) = 0 outside the box, and for continuity of the wavefunction, the boundary conditions are defined:
ψ (x) = 0, x = 0, l at the edges
therefore the allowed energies are integer numbers of half wavelengths of the sine wave
1st part of particle in a box derivation using the boundary conditions
at x = 0, ψ = 0. A = 0 is not an allowed condition so sin(kx+ϕ) = 0
x = 0, so Asin(ϕ) = 0
this is satisfied by ϕ = nπ
at x = l, ψ = 0.
Asin(kl) = 0 → sin(kl) = 0 → kl = nπ → k = nπ/l
ψ (x) = Asin((nπ/l)x) when 0 ≤ x ≤ l
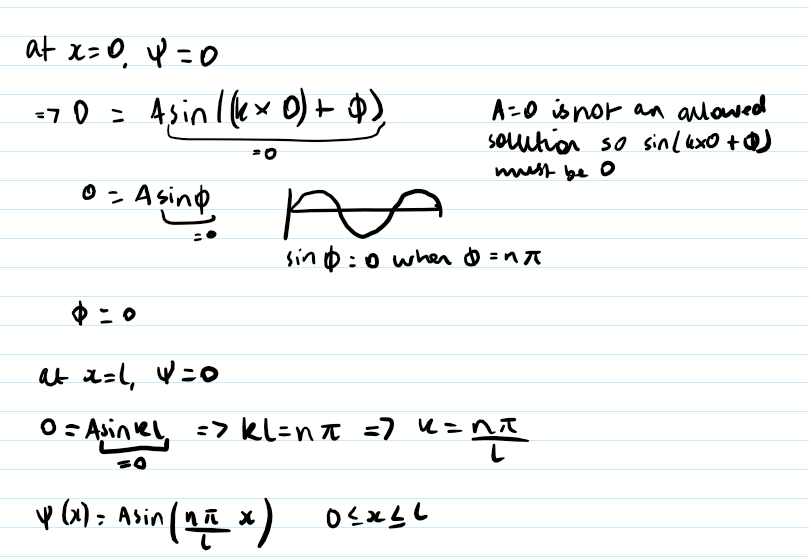
equation for En and how is it derived from particle in a box
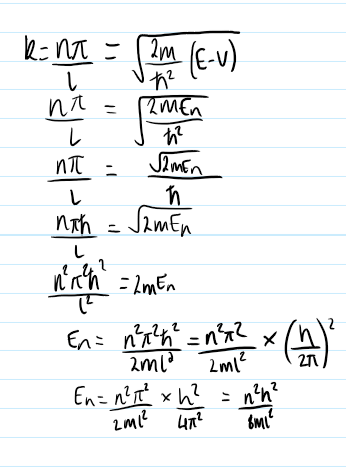
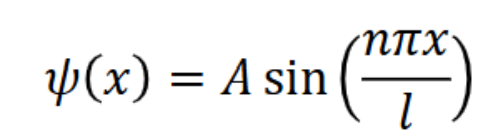
how is A found and how can the equation for ψ be expressed as a result?
know particle is in box so set probability to 1.
substitute in the equation for ψ
use the identity for sin2(ax)
solve to find A
put A back into equation for ψ

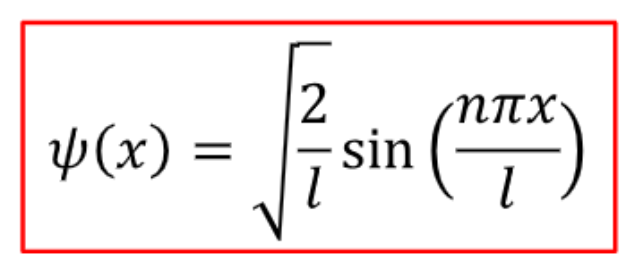
full derivation (only do after know other flashcards)

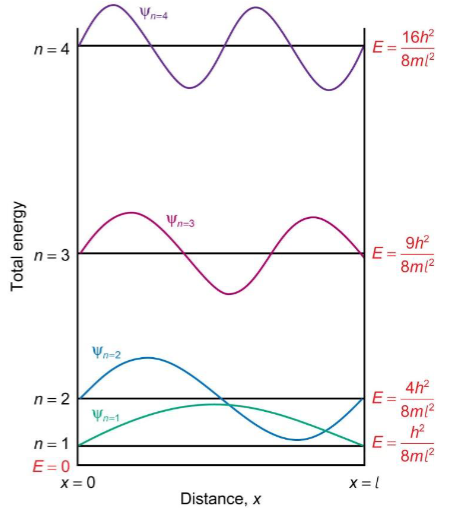
n = 1 to n = 4 particle in a box diagram including equations for E
how can the equation for En be derived from the de Broglie wavelength
inside the box, the potential energy, V, is zero, so energy is all kinetic
substitute v from kinetic energy into de Broglie equation
use the allowed wavefunctions formulae and sub in wavelength
rearrange to get equation for En - agrees with Schrodinger approach
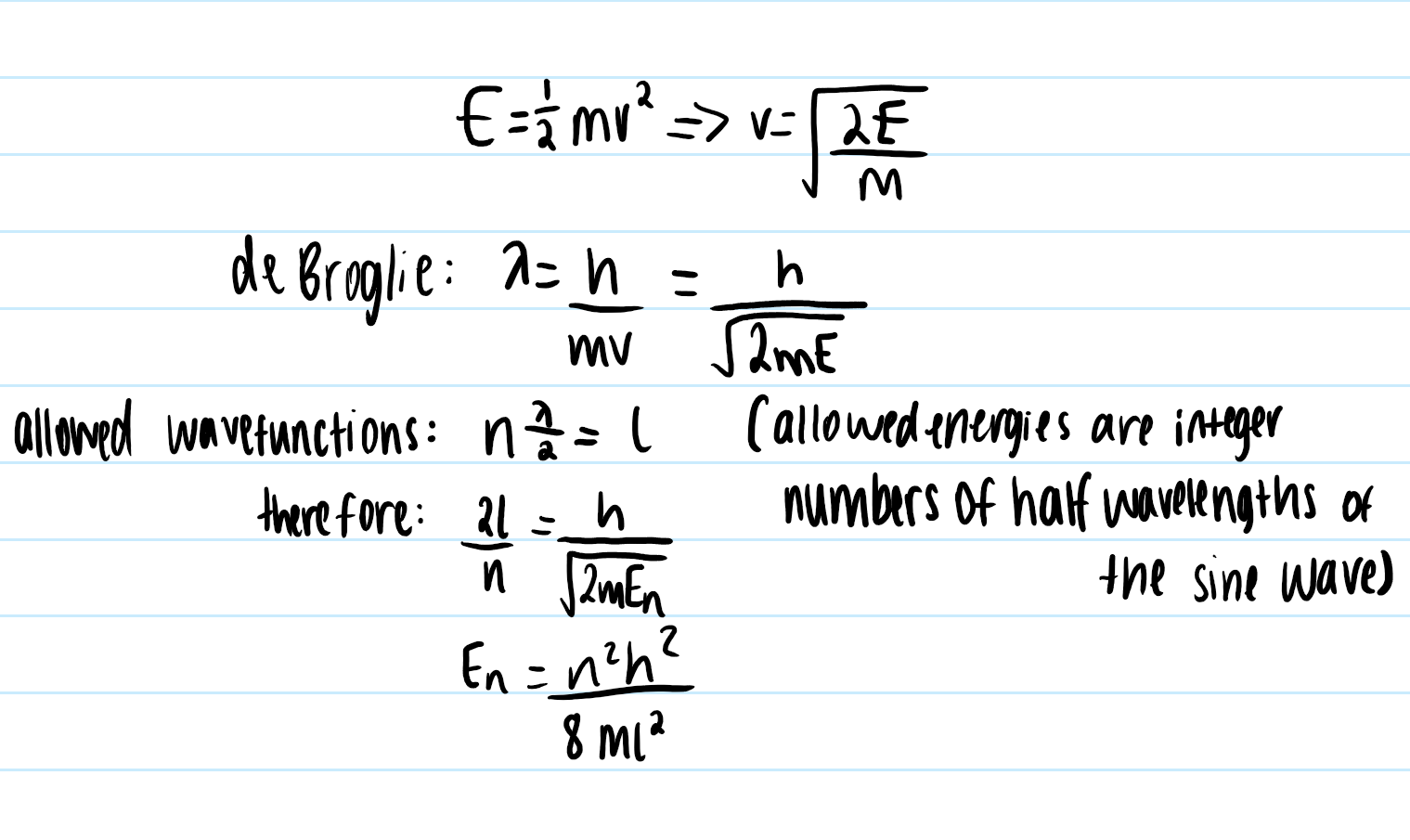
Etranslation for 3D particle in a box
3D box with sides lx, ly and lz
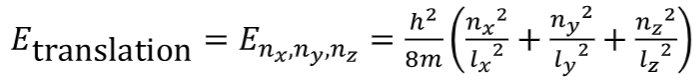
lowest energy transition and approximate ΔE for He atom in a cubic box of side 1m
comment on value of ΔE and what this means
lowest energy transition is from E1,1,1 → E2,1,1 (= E1,2,1 = E1,1,2)
ΔE ≈ 2.48 × 10-41 J/molecule - so tiny and so transitions between translational energy levels are too small to measure, meaning translational motion can be treated as a classical continuum
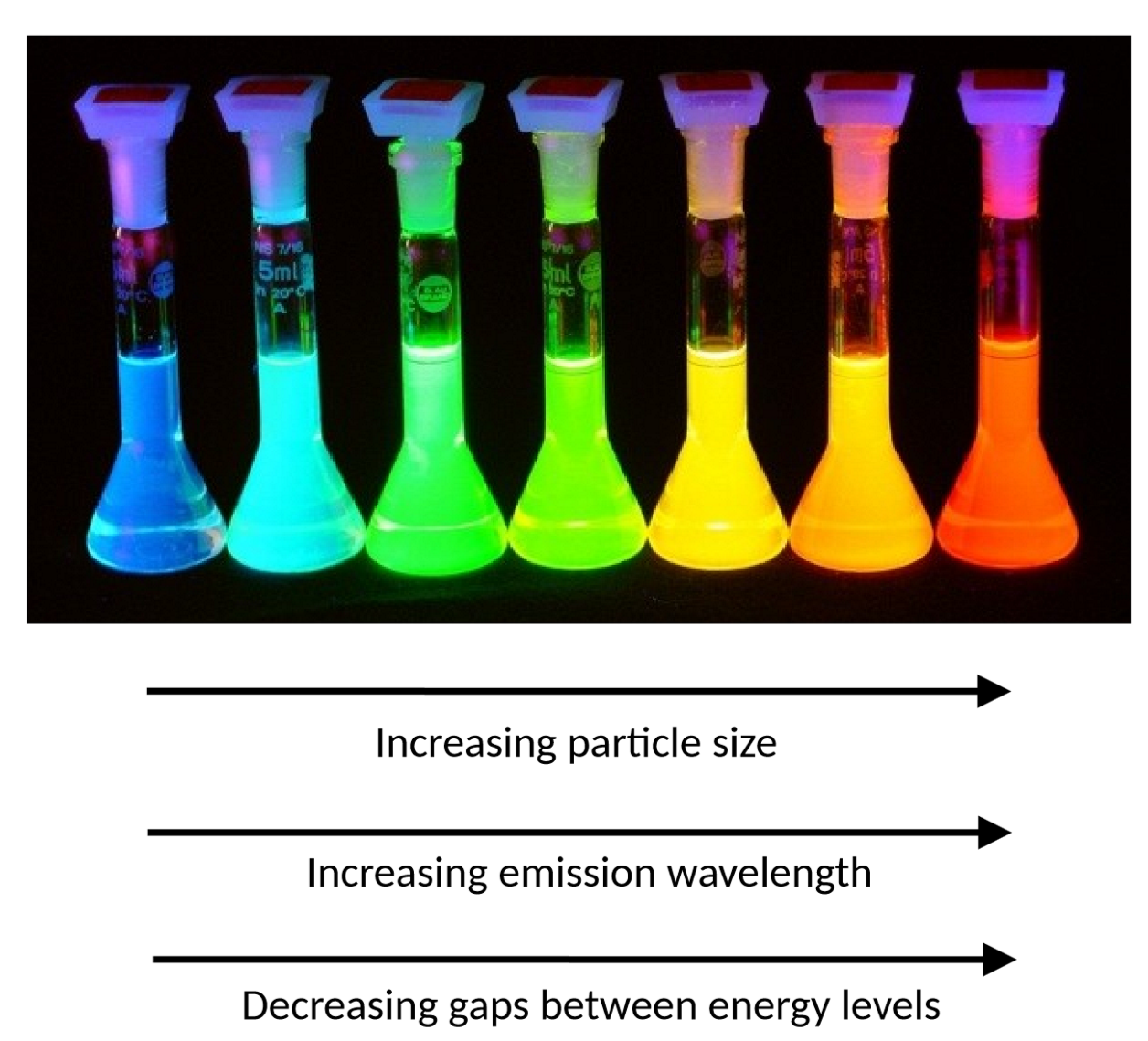
what are quantum dots
semiconducting, fluorescent nanoparticles ranging from ~2-10 nm in diameter. they emit visible light. electrons are confined to the particle so energy levels can be modelled using 3D particle in a box

emission wavelength, ΔE and particle size from left to right