Carbon and Functional Groups
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Carbon
A tetravalent (4 valence e- = able to form up to four bonds) atom that is essential to forming organic (carbon-containing) molecules
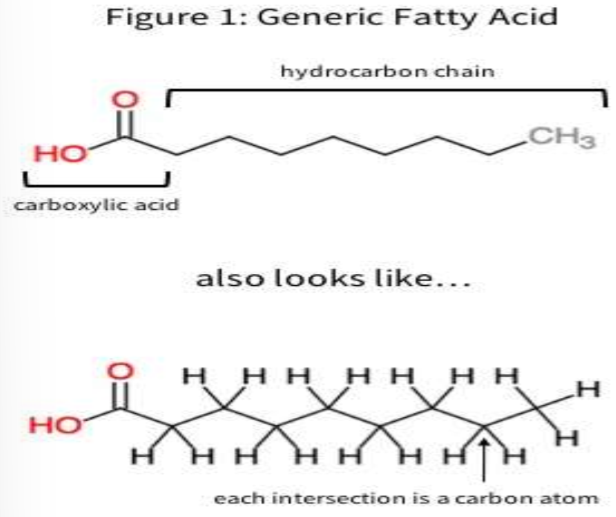
Hydrocarbons
Organic molecules consisting of only carbon and hydrogen (ex. fossil fuels, fat hydrocarbon tail)
Functional group
A group of atoms responsible for the chemical characteristics of a particular compound
What are the 6 functional groups you need to know + mnemonic?
Happy Cats Always Make Perfect Snacks -
Hydroxyl
Carboxyl
Amino
Methyl
Phosphate
Sulfhydryl
Hydroxyl
Chemical formula: OH
Polar because of oxygen’s electronegativity
Makes substances soluble
Found in sugars and alcohols

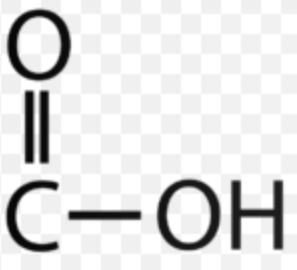
Carboxyl
AKA Carboxylic acid
Chemical formula: COOH
Found in amino acids
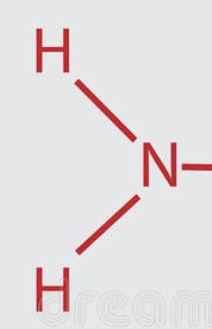
Amino
AKA Amine group
Chemical formula: NH2
Found in bases and amino acids
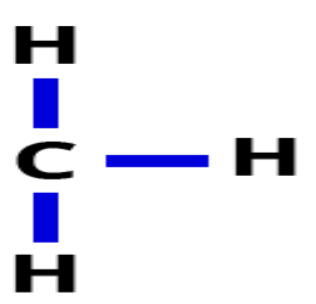
Methyl
Chemical formula: CH3
Nonpolar
Used by cells to alter expression rates of certain genes
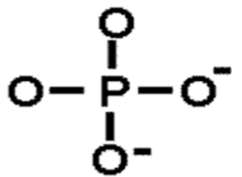
Phosphate
Chemical formula: PO42-
Negative charge = hydrophilic properties
In nucleotides, phospholipids, ATP
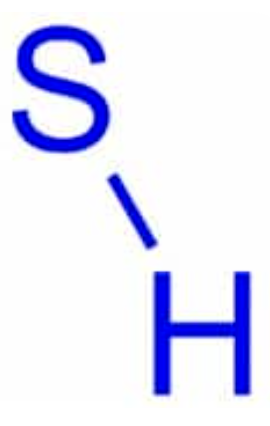
Sulfhydryl
Chemical formula: SH
Holds things into place, helps with rigidity
Found in crosslinked molecules, like rubber and hair