Primary and secondary structures
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Describe the primary structure of a polypeptide
Amino acid sequence in polypeptide chain
Linked by peptide bonds
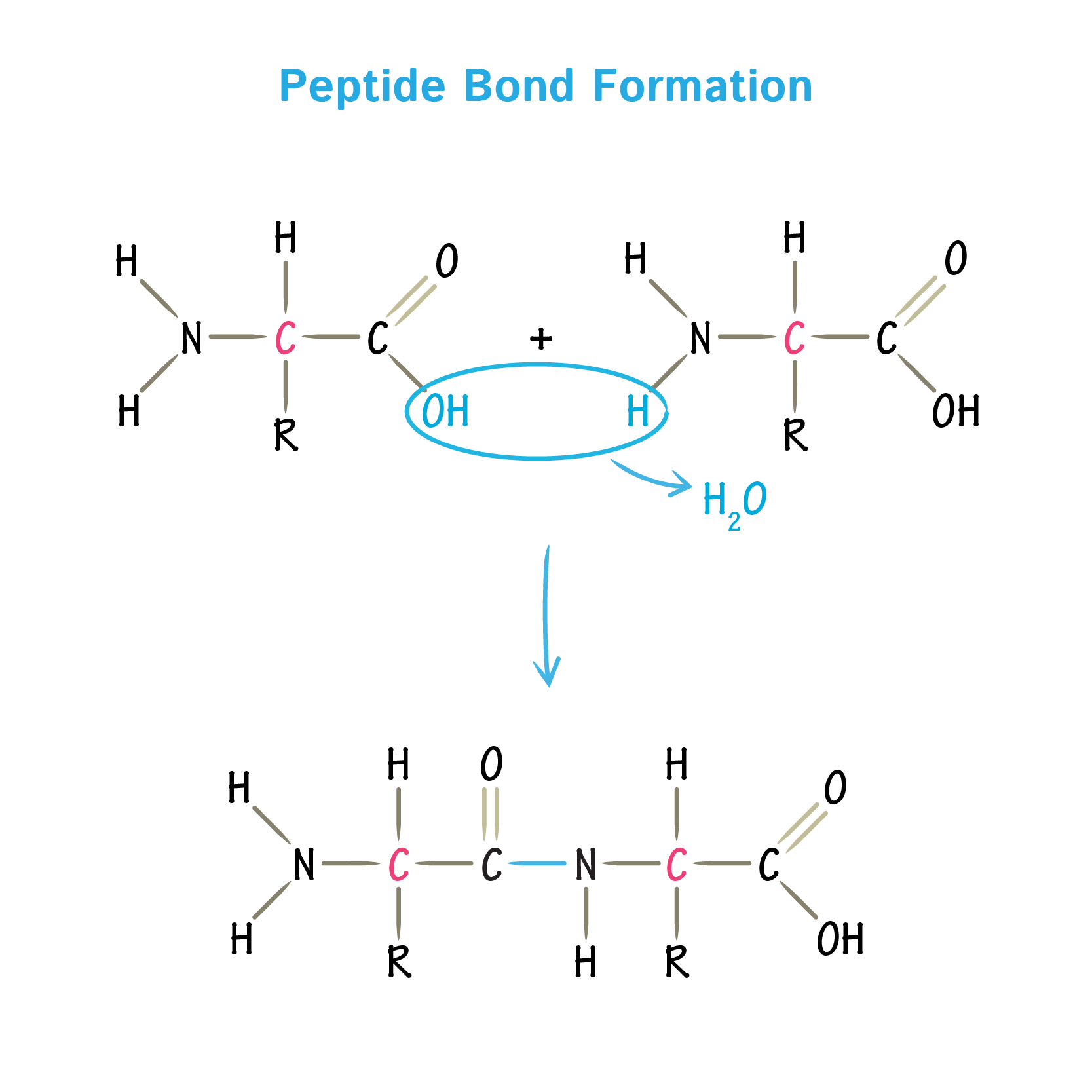
Describe properties of a peptide bond
Have some features of a ‘double bond’
Shorter C-N bond length
Delta negative oxygen
Delta positive nitrogen
Resonance
Occur when a double bond is next to another bond that has the potential to be a double bond
Shared electrons in a covalent bond fluctuate
Go between the C=O & C=N bond
Rigid C-N bond = no rotation around the peptide bond
Planar
Side chains alternate on different sides of the polypeptide chain
Side chains alternate
Side chains have fixed orientation
Trans arrangement - don’t clash
Explained using the Ramachandran plot
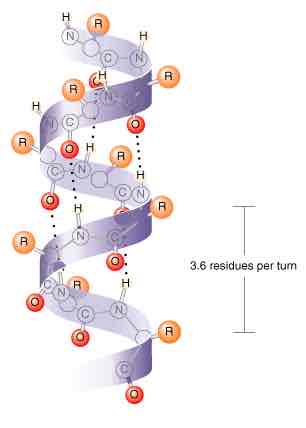
Describe the secondary structure
Hydrogen bonding
Form alpha helix and beta pleated sheets
Alpha helix
H bonds in the same polypeptide chain
Between oxygen on C=O and hydrogen on N-H
Every 4th peptide bonds
3.6 residues per turn - verticals length of 1 full turn (pitch) = 0.54nm
R-groups point outwards
Rigid cylinder shape
Describe the tertiary structure of the polypeptide
Overall structure of the folded polypeptide chain
Ionic bonds
Disulphide bridges
Hydrogen bonds
Describe the quaternary structure of the polypeptide
2 or more folded polypeptide chains
Assembly of subunits