Lecture 04: Ca2+ Signalling in Health and Disease
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
What Are Arachidonate Regulated Ca2+ (ARC) Channels?
Non-store operated Ca2+ channels → no regulated by ER Ca2+ store depletion
Regulated by the secondary messenger Arachidonic acid
Diverse Ca²⁺ channels that are involved in sensing the cellular environment.
Discovered in Drosophila, initially linked to visual transduction.
Electrophysiological recordings showed transient potentials in the retina in response to light.
Describe the Experiment Used to Conceptualise Ca2+ Store-operated Ca2+ Entry
Cytosolic [Ca2+] measured over time using a Ca2+ indicator
A high concentration of agonist is applied → generates peak (inital Ca2+ release) and plataue (sustained Ca2- release) response,
This sustained Ca2+ release is due to Store operated Ca entry, mediated by SOCE or CRAC
What is the difference between capacitative and non-capacitative Ca²⁺ entry, and how do they relate to Ca²⁺ oscillations?
Stimulation with low concentrations of agonist generates Ca²⁺ oscillations (non-capacitative Ca2+ entry)
External Ca²⁺ removal/ reduction in some cells (e.g., epithelia) affects the frequency of oscillation but doesn’t stop the oscillations until ER stores are depleted.
Capacitative (store-operated) Ca²⁺ entry → triggered by ER store depletion.
Non-capacitative Ca²⁺ entry → maintains and controls the frequency of Ca²⁺ oscillations even when stores aren’t depleted (not store depletion dependent)
Oscillations can persist without Ca²⁺ entry, but frequency depends partly on non-capacitative Ca²⁺ entry,
Do ER Ca²⁺ stores fully deplete during cytosolic Ca²⁺ oscillations in pancreatic acinar cells?
During low agonist stimulation (e.g., ACh), ER Ca²⁺ stores are not maximally depleted.
Cytosolic Ca²⁺ oscillations occur without triggering store-operated Ca²⁺ entry (SOCE).
High agonist concentrations (~10 μM ACh) are required for substantial ER Ca²⁺ depletion, leading to a large, sustained cytosolic Ca²⁺ increase.
This was demonstrated using the ER loaded with a low-affinity Ca²⁺ dye to detect high [Ca2+]; patch-clamp measurements of Ca²-dependent Cl⁻ currents served as a proxy for cytosolic Ca²⁺.
How are cytosolic Ca²⁺ oscillations sustained if ER Ca²⁺ stores are not fully depleted?
Repetitive cytosolic Ca²⁺ oscillations occur even with no extracellular Ca²⁺.
Low agonist concentration (ACh) activates mAChRs, which causes Ca²⁺ release via IP3
SERCA pumps resequester Ca²⁺ into the ER, delaying store depletion.
Experiment with no [Ca2+]o: Only after prolonged stimulation or high concentrations of agonist doses do stores deplete enough to trigger SOCE → corresponds to sustained increase in Ca2+
Suggests intrinsic IP3-mediated Ca2+ release + ER resequestration via SERCA* can sustain oscillations independently of extracellular Ca²⁺ entry.
*Takes longer for stores to be depleted and to activate SOCE
What Maintains Cytosolic Ca2+ Oscillations At Low Agonsit Concentration?
Non-capacitative Ca²⁺ entry maintains oscillations when ER stores are not fully depleted.
Most of the Ca²⁺ released is resequestered into the ER via SERCA, but some is pumped out by PMCA, and so requires a small amount of Ca²⁺ entry to maintain Ca2+ oscillations
This small Ca²⁺ entry helps control the frequency of cytosolic Ca²⁺ oscillations
Arachidonate-Regulated Ca²⁺ (ARC) channels mediate this Ca²⁺ entry, activated by GPCR-induced arachidonic acid signalling.
What is the evidence for arachidonic acid (AA) as a secondary messenger for non-capacitative Ca²⁺ entry?
Exogenous AA (3–8 mM) induces Ca²⁺ entry without store depletion (measured via fluorescent dye or patch clamp).
Agonists (low conc.) activate PLA₂, which synthesises AA.
Inhibiting AA synthesis prevents agonist-evoked Ca²⁺ entry.
AA’s effects on Ca²⁺ entry are independent of its metabolism
What features are shared between CRAC (SOCE) and ARC (AA) channels?
Both are inwardly rectifying
Very positive reversal potential
Similar IV curves
Highly Ca²⁺ selective
Blocked by trivalent cations (e.g., La³⁺)
How do CRAC (SOCE) and ARC (AA) channels differ in activation and regulation?
Activation:
CRAC → activated by store depletion and ER STIM1
ARC → activated by arachidonic acid and PM STIM1
Kinetics & regulation:
ARC: inhibited by high internal Ca²⁺, but not low external pH and 2-APB
CRAC: not inhibited by high internal Ca²⁺, but is inhibited by low pH and blocked by 2-APB
ARC currents are not fast-inactivating; CRAC can show fast inactivation
Where is STIM1 located and what does its name indicate
Majority is located in the ER membrane; a small fraction in the plasma membrane
Name = Stromal Interacting Molecule 1 (discovered in a screen interacting with external environment)
What is the Molecular Identity of the ARC Channel and How Was it Discovered?
Molecular identitiy: Heteropentamer of Orai1/Orai3 , with atleast two Orai3 subunits
Determined using concatenated constructs: every possible subunit composition (stuck C- and N-terminal domains of subunits together to make a single cDNA piece encoding every possible permutation of the channel)
Only heteropentamers containing 2 Orai3 subunitss produced roubsut AA-induced Ca2+ currents
Store depletion failed to induce substantial currents in cells expressing these channels
How does the subunit composition of Orai channels determine ARC vs CRAC function?
ARC channels and currents: 2× Orai3 + 3× Orai1 subunits→ activated by arachidonic acid, not store depletion; no response to thapsigargine
CRAC-like channels and currents: 4× Orai1 subunits → respond to store depletion
Demonstrates that subunit composition dictates channel type and activation mechanism
How were ARC Channels Shown to Be Regulated by Arachidonic Acid and Plasma Membrane STIM-1?
Demonstrated using recombinant DNA techniques, where STIM-1 was engineered to traffic to the plasma membrane
Increased PM STIM-1 enhanced ARC currents, confirming its role in channel activation
How Do ARC Channels Contribute to Physiological Ca2+ Oscillations?
During low agonist stimulation, there is cyclical ER Ca²⁺ release and reuptake generating Ca oscillations, but there is insufficent store depletion to activate SOCE.
Small amounts of Ca²⁺ release from the ER generate Ca2+ spikes, then reuptake into ER maintains Ca²⁺ oscillations.
ARC channels, activated by arachidonic acid (AA) at low agonist concentrations, facilitate and sustain Ca²⁺ oscillations, helping preserve ER Ca²⁺ integrity.
How Do ARC Channels Contribute to Pathological Ca2+ Oscillations?
High concentrations of agonist cause a substantial release of Ca and ER store deleption → sufficient to activate SOCE and replenish ER stores → generates high cytosolic Ca2+
This high sustained Ca signal inhibits the ARC channels, switching them off
In most cells, this sustained Ca²⁺ signalling is considered pathological, potentially contributing to disease
What is the hypothesis of Reciprocal Cross Talk Between SOCE and ARC Channels?
Low agonist concentrations: Activate ARC Channels → sustained Ca oscillations; insufficient ER Ca store depletion → SOCE NOT ACTIVATED
ARC is responsible for physiological Ca oscillations
Increasing agonist concentrations: increased frequency of Ca oscillation → fuse to sustained increase in cytosolic Ca2+ (pathological
ARC channels are inhibited.
Store depletion sufficient → activation of SOCE (SOC channels take over)→ plateau phase → pathological Ca overload response (contributes to disease
Likely, many ARC and SOC-like channels are implicated
Contribution from STIM and ORai1/3 subunits
Which Cells are SOC and ARC Channels involved in Ca Oscillations
SOC: Ca oscillations for T-lymphocytes
ARC: Ca oscillations for epithelial cells
How Do ARC Channels Drive Further Ca Oscillations?
Evidence that Ca entry through ARC channels activates PLCδ drives further Ca oscillations
PLCδ is a Ca-dependent PLC subtype → drives generation of further IP3, accentuating Ca2+ oscillations
Supports idea that ARC mediated Ca entry is important in mediating physiological Ca2+ oscillatiosn
What are Transient Receptor Potential (TRP) Channels and How Are They Classified?
A large family of ion channels, subdivided into distinct families with distinct functions.
Classification:
TRPC (Classical / Short TRP)
TRPM1-8 (Melastatin / Long TRP)
TRPV1-7 (Vanilloid TRP)
TRPML (Mucolipins)
TRPP (Polycystins)
TRPA1 ANKTN1s
Latter 3 - most functionally obscure and diverse members
Dendrogram branch lengths show evolutionary diversity and mutational differences between members
Describe the Structure of the TRP Channel:
Tetrameric ion channels → composed of 4 subunits forming a central pore
Selectivity filter: Formed by pore loops, one from each subunit
Amino acids dip into the lipid bilayer to determine ion permeability
S6 transmembrane helix: acts as the gating helix → conformational changes open/close the channel
TRP box domain (located at the C-terminal)
consists of the conserved sequence (e.g. EWKFAR in TRPC)
Less conserved in TRPV and TRPM channels
Couples gating of S6 to channel activation
What Regulatory and Family-Specific Domains Are Found in TRP Channels?
Coiled-coil (CC) domains: Mediate subunit assembly and channel stability
Ankyrin repeats (AnkR) range from 0–14 repeats depending on family
~3–4 in TRPC/TRPV, up to 14 in ANKTM
CIRB domain: Putative calmodulin- and IP₃ receptor–binding region
EF-hand domain: canonical Ca²⁺-binding helix–loop–helix motif
PDZ-binding motif: Enables protein–protein interactions (e.g. scaffolding proteins)
Wha Family-Specific Domains Are Found in TRP Channels?
PLIK domain (TRPM6/7):
phospholipase-C-interacting kinase, an atypical protein kinase intrinsic to the TRPM6 and TRPM7 polypeptide chains
Independent of ion conduction
Nudix domain (TRPM2):
NUDT9 hydrolase protein homologue found in TRPM2
Binds ADP-ribose → channel activation
Describe the Structure and Function of the TRPC (Classical/ Canonical) Family Channels
Two main groups
TRPC1, TRPC4 & TRPC5
TRPC3, TRPC6 & TRPC7 → activated by DAG → receptor operated non-selecive cation channels
TRPC2
In humans: a pseudogene → Acquired mutations over time and functionally redundant
in rodents: (mTRPC2) → Involved in pheremone sensing
What is the Vomeronasal Organ (VNO)?
The first stage of the accessory olfactory system → contains sensory neurons that detect pheromone chemical stimuli
Pheremones: chemical messengers carrying information between individuals of the same species
Neuronal axons project to the accessory olfactory bulb, targeting the amygdala and bed nucleus of the stria terminalis, which in turn project to the hypothalamus.
Important in social and sexual behaviour → linked to complex emotional and behavioural responses, including sexual behaviour, sexuality, aggression, anxiety and fear.
Well studied in mice and animals
Why is the Vomeronasal Organ Not Well Studied in Humans?
Anatomy in humans is poorly defined → sggested that the organs function regresses during foetal development
Many genes essential for its function in animals, e.g. TRP2C are non-functional in humans
Its function in humans is of contention
What is the Intruder Assay?
A well-validated behavioural assay of male-type social and sexual behaviour in rodents, e.g. mice
Mice raised together with their siblings in the same cage (get along)
The resident male is kept alone for a period of time
If a male Intruder mouse is introduced into the same cage,→ two males will fight → pheromones induce aggressive fighting behaviour
If a female intruder mouse is introduced into the same cage → mating behaviour
Used to study pheromone-dependent social behaviour
How Does the Intruder Assay Demonstrate the Role of the Vomeronasal Organ (VNO)?
Surgical removal or genetic disruption of the VNO in male rodents:
Abolishes or greatly reduces
Male–male aggression
Male–female mating behaviour
Indicates that:
Male and female pheromones are detected by the VNO
VNO signalling is essential for pheromone-driven social behaviours
Confirms the VNO as a key pheromone-sensing organ in rodents
How Was TRPC2 Implicated in Pheromone Sensing by the VNO?
Immunohistochemistry and immunofluorescence of the VNO showed TRPC2 channels localised to the apical lumen of the VNO epithelium, suggesting t
No other TRP channels were detected in these cells
This localisation and selective expression suggest that TRPC2 is the candidate transduction channel responsible for sensing pheremones
What Was Observed in the Intruder Assay Follow Up Study Using TRPC2 KO Mice?
TRPC2⁻/⁻ mice show normal development
Normal lifespan, fitness, litter size, and baseline behaviour
Introduction of WT resident male to intruder WT male or TRPC2⁻/⁻ male → fighting behaviour
Introduction of TRPC2⁻/⁻ resident male + any intruder (WT female, WT male, castrated male, or urine-sprayed castrated male) → mating behaviour
Indicates failure to discriminate male pheromones
What Do TRPC2 Knockout Studies Reveal About Pheromone Sensing and Behaviour?
TRPC2 in the VNO is required to detect male urine pheromones → confers mouse sex recognition
Detection of male pheromones normally triggers aggression/fighting behaviour
In TRPC2⁻/⁻ mice:
Male pheromones are not detected
The default behavioural response becomes mating, regardless of the intruder's sex
Demonstrates TRPC2’s role in sex recognition and pheromone-driven social behaviour
Findings attracted public/media attention due to perceived relevance to human behaviour → not directly translatable)
What did TRPC2 Gene Sequencing in Primates and Monkeys Reveal?
When compared to numerous species across the evolutionary timeline, there were increasing numbers of mutations gained along the evolutionary timeline, leading to non-functional TRPC2 in monkeys/primates and higher organisms e.g. humans
Suggested that the development of colour vision and visible secondary sexual characteristics has largely replaced pheromone signalling → no longer rely on TRPC2 to sense pheremones
What are the Key Features of TRPV1 Channels?
activated by heat (>43ºC),
activated by capsaicin (Chilli)
activated by pH <6 (Acidic)
non-selective cation channels →Ca2+ permeable
What are the Key Features of TRPV2/3 Channels?
activated by heat at a lower threshold (>31ºC),
not capsaicin activated
non-selective cation channels → Ca permeable
What are the Key Features of TRPV4 Channels?
Activated by heat at a low threshold (>25ºC),
Activated by hypotonicity (“acts as osmosensor” to changes in cell volume),
May be important nociception? (sensation of pain)
Non-selective cation channels → Ca permeable
What are the Key Features of TRPV5/6 Channels?
highly Ca2+-selective!
involved in transepithelial Ca2+ absorption in the kidney and bone
Involved in the unidirectional movement of Ca2+
Aka ECaC1/CaT2 & ECaC2/CaT1
How Was The TRPV1 Channel Discovered?
Discovered by David Julius using an expression cloning strategy, based on capsaicin-induced Ca²⁺ influx
A rat dorsal root ganglion (DRG) neuron cDNA library wase used
was expressed in a heterologous cell line → used to identify a single cDNA clone, encoding TRPV1
cDNA fragments were sequentially expressed in a heterologous cell line
Cells were screened for capsaicin-evoked Ca2+ responses until capsaicin sensitivity was detected
A single cDNA clone encoding TRPV1 was identified
Responses to chilli extracts of increasing hotness (e.g. poblano → habanero) correlated with capsaicin sensitivity
What are the Functional Properties and Physiological role of TRPV1?
A non-selective, Ca²⁺-permeable cation channel
Activated by:
Capsaicin
Noxious heat
Structurally related to the TRP ion channel family
Capsaicin evokes a large inward Ca²⁺ current
Channels sense noxious stimuli →strong activation can cause sensory neuron death
Explains desensitisation to spicy food (eating lots of spicy food → become immune)
Channel functions as a molecular transducer of painful thermal and chemical stimuli in vivo
What Are the Key Features of the TRPM (Melastatin) Family Channels 1, 2, 5, and 8?
TRPM1: Tumour suppressor
TRMP2: potential “redox/ metabolism sensor’ → activated by ADP-ribose, H202, NAD+
important co-factors in metabolism generated in oxidative environments
TRMP5: Coupled to taste receptor signal transduction (T1R, T2R)
TRMP8: activated by cold (<30ºC) and menthol
What Are the Key Features of the TRPM6/7 (Melastatin) Family Channels?
They are chanzymes → have channel and enzyme activity
Kinase enzyme domain function is independent of the Ca/Mg channel activity
Ca2+ and Mg2+ permeable
Involved in body Mg2+ homeostasis
Involved in cell growth/ proliferation and cell migration
What is the Structure and Transmembrane Topology of The TRPM2 Channel?
A six-transmembrane (S1–S6) non-selective cation channel
Pore loop located between S5–S6
N-terminus:
Four MHR (melastatin homology regions) of unknown function
IQ-like calmodulin-binding motif → involved in Ca²⁺ regulation
C-terminus contains:
TRP box
Coiled-coil domain
NUDT9-H (Nudix homology) domain → ADP-ribose (ADPR) binds to allow influx of Ca²⁺ and Na
Both N- and C-termini face the cytosol
ADP-ribose (ADPR) binds to the NUDT9-H domain to gate the channel
How is TRMP2 Gated and Regulated?
ADP-ribose (ADPR) binds the NUDT9-H domain → opens the channel, allowing Ca²⁺ and Na⁺ influx.
Gating is facilitated by H₂O₂, cyclic ADPR (cADPR), and Ca²⁺.
ADPR is hydrolysed to AMP and ribose-5-phosphate by the NUDT9-H domain.
AMP and 8Br-cADPR act as negative regulators of TRPM2 gating.
What is the Signalling Mechanism for TRMP2 Activation
NAD⁺ and reactive oxygen species (ROS, e.g., H₂O₂) accumulate during inflammation and tissue damage.
Extracellular NAD⁺ is converted to ADP-Ribose, cADP-Ribose, and NAADP by ectoenzymes CD38/CD157.
Extracellular ADPR binds plasma membrane receptors (e.g., P2Y) and increases [Ca2+] through G-protein and PLC activation, leading to IP₃ generation and Ca²⁺ release from stores.
H₂O₂ can cross the membrane and mobilise ADPR from mitochondria
cADPR and H₂O₂ can synergise with ADPR to activate TRPM2.
Free ADPR generated from poly-ADP ribose (via PARP-1 and PARG) during ROS-induced DNA damage also activates TRPM2.
What is the Functional Effect of TRMP2 Activation?
Free cytosolic ADPR binds NUDT9-H on the plasma or lysosomal membrane → Ca²⁺ influx across PM or lysosomal Ca²⁺ release
This increases cytosolic Ca²⁺ and contributes to redox sensing, neuronal signalling, and possibly neurodegeneration
Neurodegeneration is thought to be a functional consequence of redox and oxidative stress-mediated response
Ca²⁺ overload can trigger apoptosis or necrosis.
Other extracellular signals may produce free intracellular ADPR to gate TRPM2 channels at the Plasma membrane/ lysosomes and regulate receptor-mediated signalling
Summary of Temperature Sensitive TRP Channels
TRPM8 and TRPV neurons are all expressed in sensory neurons and other cell types e.g. keratinocytes (pain sensing) and hypothalamus (body temp control), etc
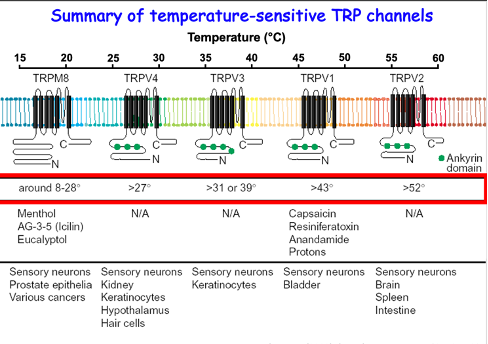
What are the TRPP (Polycystin) Channel Family?
Discovered following an analysis of mutated genes from polycystic kidney disease (PKD)
Non-selective Ca2+-permeable cation channels are important for
epithelial cell function, e.g epithelial polarity (kidney and pancreas)
Embryonic development (cilia movement in embryonic ventral node → guides embryo development and wbody asymmetry)
Two main channels
PKD1 (TRPP1): 11 TMD proteins, with 6 forming the channel pore; mutated in PKD
PKD2 (TRPP2) 6 TMD proteins forming the channel pore; mutated in PKD
What Disease Are Associated With Mutations in Specific TRP Channels?
TRPV4: Skeletal dysplasias → umbrella term to hundreds of syndromes affecting bone/cartilage growth
TRPML: Mucolipidosis type IV → autosomal recessive lysosomal storage disorder; delayed psychomotor development, ocular issues (corneal opacity, retinal degeneration)
TRPM1: Congenital stationary night blindness → reduced visual acuity, defective dark adaptation
TRPM2: Bipolar disorder, hereditary deafness
TRPM6: Inherited hypomagnesaemia → defective kidney Mg²⁺ reabsorption
TRPM7: Guamanian ALS / Parkinsonism dementia → impaired movement, motor coordination
TRPP1/PKD1: Autosomal dominant polycystic kidney disease
TRPA1: Familial episodic pain syndrome → peripheral neuropathy
How Do TRP Channels Function as Nociceptors in Pain Pathways?
Nociceptors present in the periphery detect heat, cold, pH changes, reactive chemicals, nerve gases, and hyperalgesia.
TRV1, V3, V4 are expressed in sensory neurons, respond to warming temperatures; TRPV2 is activated by noxious heat (physiological role unclear).
Acids and reactive chemicals are robust activators of TRPV1
Bases activate TRPA1 (key chemoreceptor that responds to reactive chemicals)
TRPM8: Detects environmental cold; also contributes to cold hyperalgesia.
TRPV1 also has a role in cold hyperalgesia
TRPV1 is expressed in CNS/DRG, relaying pain signals.
Mutations in the channel genes can alter pain perception → potential targets for analgesics (“anti-nociceptives”).
Activation of TRP cation channels triggers action potentials in sensory neurons; mostly involved in sensing, not emotional pain.
What Progresses Has Been Made With TRPV1 Channels as Novel Theraputic Targets for Analgesia?
A few drugs that target the channel as a treatment for analgesia have been effective
Little to non have reached the market due to adverse side effects
What is the Role of TRP Channels in the Bladder?
Micturition reflex mediated by TRPV1-positive nerves (and TRMP8-positive nerves)
These same neurons convey nociceptive information, e.g. bladder pain (cysitis), to the CNS
TRPV4 is important in bladder function, present on urothelium and detrusor muscle; activated by bladder distension (stretch) and hypo-osmolar urine
Micturition reflex is controlled descending CNS pathway; CNS disruption, e.g. spinal cord injury or MS causes this to become autonomous and partially driven by TRPV1
How Do TRP Channels in the Bladder Represent a Therapeutic Target For Benign Prostatic Hyperplasia, Micturition or Overactive Bladder Syndrome?
TRPV1 antagonists or desensitisation → reduce painful bladder disorders / BPH-related pain.
TRPM8 agonists → potential therapy in overactive bladder and pain induced by benign prostatic hyperplasia
TRPV4 blockers → potential therapy for overactive bladder.
What is the Role of TRP Channels in the Skin
TRPV1 expression by various cell types (e.g, sebocytes, keratinocytes, sensory neurons and cells of the hair follicles)
TRPV1 activation induces heat sensation and the development of skin-derived pruritus, and suppresses sebaceous lipid synthesis
TRPV1 and TRPV3 activation:
shift the proliferation-differentiation balance of epidermal keratinocytes towards differentiation
increases pro-inflammatory cytokine release on epidermal and hair follicle-derived keratinocytes → important in situ immunoregulation of skin)
regulates hair cycle directly (and indirectly via TRPV1-induced follicular growth factor production)
Target of TRPM3 for male pattern boldness
TRPV1,2,3,4,6 activation is involved in the regulation of epidermal barrier formation
Synergistic effect with TRPA1 and TRPM8 (also involved in maturation and differentiation of keratinocytes)
TRMP7 regulates melanogenesis of melanocytes → target for skin cancer
How Do TRP Channels Serve as Therapeutic Targets in the Lungs and Airways?
Sensory nerves (vagal terminals): TRPA1 & TRPV1 → activated by noxious chemical and physical stimuli → cause nerve activation → initiate reflexes & sensations (coughing, chest tightness).
Airway smooth muscle: TRPC3 & TRPV4 → contribute to Ca²⁺-mediated smooth muscle constriction → airflow obstruction → targets in asthma/bronchoconstriction.
Airway vascular smooth muscle: TRPC6 → mediates vessel constriction → reduces blood flow.
Endothelial cells: TRPC1, TRPC4, TRPV4 → activation increases vascular permeability → fluid leakage into interstitial space → airway oedema
Theraptuic implications for asthma
Alveolar macrophages: TRPV4 activation triggers ROS/RNS production (toxic) and TRPV2 activation stimulates phagocytosis.
Clinical data supporting the TRP channel role in respiratory sensory nerves
TRPA1 & TRPV1 agonists (tear gas, pepper spray) → acute exposure causes intense incapacitating respiratory irritation.
Potential therapeutic targets for cough, bronchoconstriction, airway oedema, and asthma.
What is Autosomal Dominant Polycystic Kidney Disease (ADPKD)
The development of fluid-filled cysts of epithelial origin in kidneys (and sometimes pancreas).
Caused by mutations in polycystins PKD1 (TRPP1) and PKD2 (TRPP2).
Cellular effects:
Epithelial de-differentiation & loss of polarity
Increased cell–matrix and cell–cell adhesion
High proliferation & apoptosis
Excessive fluid secretion
Results in reduced kidney concentrating ability and impaired kidney function due to numerous cysts and necrotic tissue