Chapter 15- Metabolism: Basic Concepts and Themes
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Metabolism is composed of many interconnected reactions
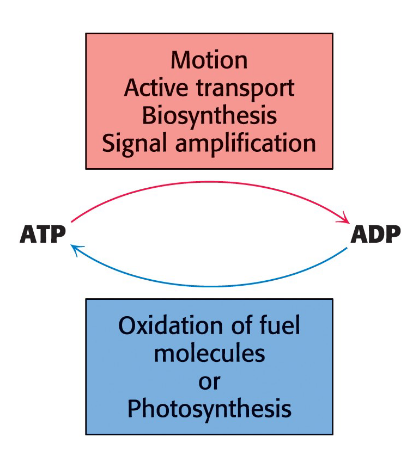
Energy is required for mechanical work (muscle contraction and cell movement), active transport, and biosynthesis.
phototrophs = capture energy from sunlight
example: all photosynthetic organisms
chemotrophs = capture energy through the oxidation of chemicals
example: all animals
have to oxidize chemical fuel to gain energy
Metabolism
Highly integrated network of chemical reactions that carry out energy extraction and synthesis of new material
Cellular processes of extracting energy and synthesizing new material are carried out by metabolism
Metabolic pathway
a series of linked reactions by which fuels are degraded and large molecules are constructed
Some reactions are not metabolically favorable
example: glycolysis is a 10-step metabolic pathway converting glucose to pyruvate
Themes common to all metabolic reactions
Metabolism is a coherent network containing many common motifs.
Adenosine triphosphate (ATP) is used as an energy currency to link energy-releasing (exergonic) and energy- requiring (endergonic) pathways.
Either sunlight or the oxidation of chemical fuels powers ATP formation.
~100 molecules serve as activated intermediates.
Metabolism uses only a few types of mechanisms that are relatively simple.
Metabolic reactions are highly regulated because metabolic pathways are interdependent.
Many of the enzymes involved in metabolism are organized into large complexes
increases speed and efficiency
allows efficient processing of unstable or toxic intermediates
Catabolism
Reactions that break down complex molecules into simpler ones to capture energy in useful forms (oxidizes)
Purpose is to generate ATP

Anabolism
Reations that construct a more complex molecule from simpler molecules by using energy

Amphibolic pathways
Pathways that can be either anabolic or catabolic depending on cellular energy conditions
A metabolic pathway must meet two criteria:
individual reactions must be specific
each of the reactions in the pathway must be thermodynamically favored under real condition
A reaction can occur spontaneously only if ∆G, the
change in free energy, is negative.
Overall free-energy change for a chemically coupled series of reactions equals…
The sum of the free-energy changes of the individual steps
allows for the coupling of thermodynamically unfavorable and favorable reactions in enzyme active sites
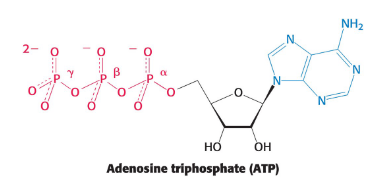
ATP
A nucleotide consisting of adenine, a ribose, and a triphosphate unit
active in complex with Mg2+ or Mn2+
Free energy derived from oxidation of food and from light is transformed into ATP
ATP acts as the free-energy donor in most energy- requiring processes
Energy-rich because its triphosphate unit contains two phsophanhydride linkages
Primary Energy Carrier
All nucleoside triphosphates are energetically equivalent
Two important electron carriers (NAD+ and FAD) and the acyl group carrier, coenzyme A, are derivatives of ATP
Is ATP hydrolysis exergonic or endergonic?
Exergonic
How does free energy get released by ATP hydrolysis?
formation of new covalent bonds.
formation of noncovalent interactions with water.
increase in entropy.
∆G for ATP hydrolysis under typical cellular conditions is approximately −50 kJ mol-1.
Enzymes catalyze the exchange of phosphoryl groups from one nucleotide to another
Some reactions are driven by GTP, UTP, and CTP.
nucleoside monophosphate kinases = enzymes that phosphorylate nucleoside monophosphates
nucleoside diphosphate kinase = enzymes that phosphorylate nucleoside diphosphates
has broad specificity
How does ATP Hydrolysis drive metabolism?
The unfavorable conversion of the compound A into compound B can be made possible by coupling to ATP hydrolysis
Renders the formation of product exergonic
Coupling these reactions under standard conditions changes the equilibrium ratio of B to A
Negative free energy
Phosphoryl Potential
Tendency of an organic molecule to transfer its terminal phosphoryl group to water
a means of comparing the tendency of organic molecules to transfer a phosphoryl group to an acceptor molecule
ex. ATP has a higher phorphoryl-transfer potential than glycerol 3-phosphate
The high phosphoryl-transfer potential of ATP can be explained by its structure
Some compounds have higher phosphoryl-transfer potential than ATP
examples: phosphoenolpyruvate (PEP) 1,3- bisphosphoglycerate (1,3-BPG), and creatine phosphate
These compounds can transfer their phosphoryl group to ADP to form ATP.
Phosphoryl transfer potential is intermediate among phosphorylated molecules → efficent carrier
Features of the ATP structure
ATP has a high phosphoryl-transfer potential because of:
orthophosphate (Pi) has greater resonance stabilization than any of the ATP phosphoryl groups
electrostatic repulsion of the triphosphate unit
negative charges repel one another because they’re close in proximity
the entropy of the products of ATP hydrolysis is greater
ADP and Pi are stabilized due to hydration.
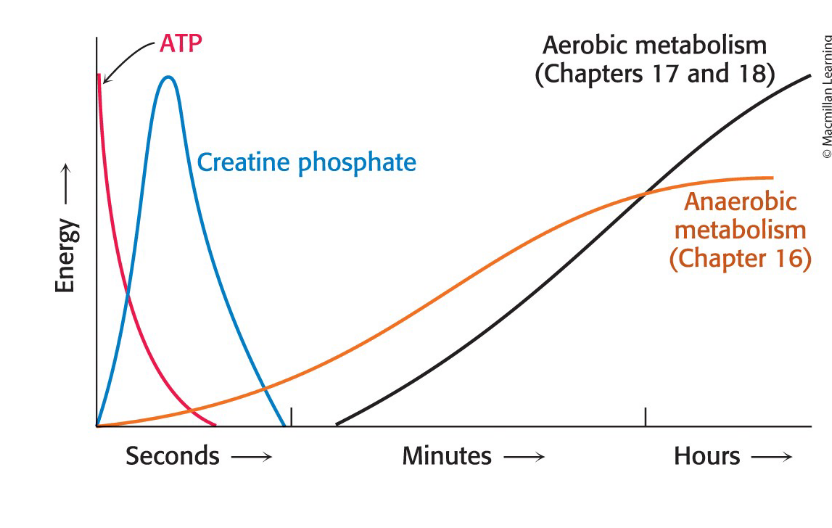
Creatine Phosphate
Serves as a reservoir of high potential phosphoryl groups
ATP in muscles sustains contractile activity for < 1 second
creatine kinase = catalyzes the regeneration of ATP from creatine phosphate and ADP
Negative free energy
How do the sources of ATP change as exercise duration increases?
Oxidation of carbon fuels
an important source of cellular energy
ATP is the principal immediate donor of free energy for biological activities, but it is limited
ATP must constantly be regenerated from ADP
Takes place one carbon at a time
carbon atoms in fuels are oxidized to yield CO2
the more reduced a carbon atom is, the more free energy is released upon oxidation
electrons are ultimately accepted by oxygen to form H2O
Are fats or carbohydrates a more efficient fuel source?
Fats- the carbon in fats is more reduced
But carbohydrates gives you the fastest energy
Glyceraldehyde 3-phosphate
A metabolite of glucose formed during glucose oxidation
the C-1 carbon is at the aldehyde-oxidation level and is not in its most oxidized state
Compounds with high phosphoryl-transfer potential can couple carbon oxidation to ATP synthesis
Oxidation of glyceraldehyde 3-phosphate does not occur directly
Carbon oxidation generates 1,3 bisphosphoglycerate (1,3-BPG), and the electrons released are captured by NAD+ to form NADH.
1,3-BPG has high phosphoryl-transfer potential, and its hydrolysis can be coupled to the synthesis of ATP
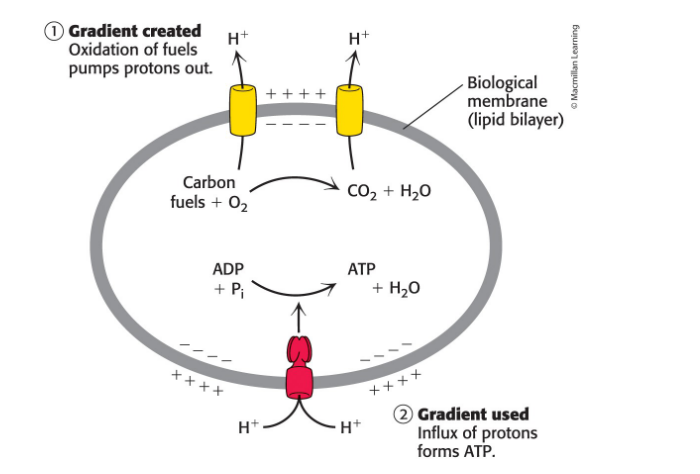
How do proton gradients power ATP synthesis
Ion gradients provide an important form of cellular energy that can be coupled to ATP synthesis
oxidation produces electrochemical potentials of ion gradients across
serves as a means of coupling thermodynamically unfavorable and favorable reactions
In animals, 90% of ATP is generated when the energy of a proton gradient is coupled with ATP synthesis
this process is called oxidative phosphorylation
Proton gradients formed using the energy from either sunlight or chemical oxidation can power ATP synthesis
Phosphates
Play a prominent role in biochemical processes
Phosphate esters are thermodynamically unstable yet kinetically stable in water.
Kinetic stability is due to the negative charges that resist hydrolysis in the absence of enzymes.
Their energy release can be manipulated by enzymes.
The addition of a phosphate group changes molecule conformation and behavior.
No other ions have the chemical characteristics of phosphate
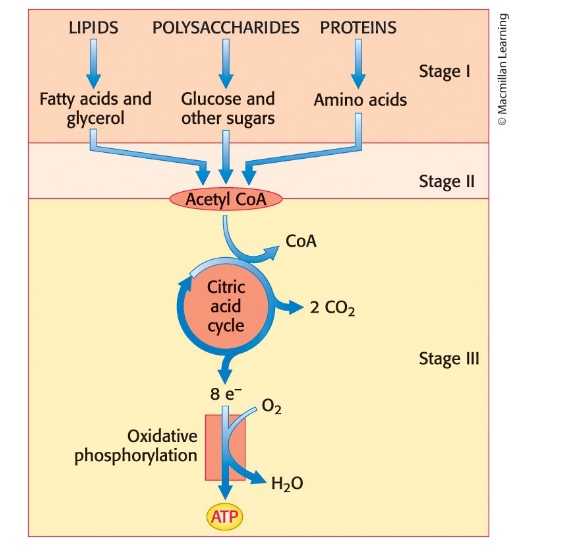
Stages of energy being extracted from food
Stage 1: Large molecules in food are broken down into smaller units (digestion)
Stage 2: Small molecules are degraded to a few simple units that play a central role in metabolism
Stage 3: ATP is produced from the complete oxidation of the acetyl unit of acetyl CoA
Activated carriers
Small molecules to which a chemical group or electrons have been added, which can then be donated to another molecule
frequently act as coenzymes or cosubstrates
example: ATP is an activated carrier of phosphoryl groups
NADH
an activated carrier of electrons for fuel oxidation
Fuel molecules transfer electrons to carriers, which then transfer their high-potential electrons to O2.
nicotinamide adenine dinucleotide (NAD+) = accepts a proton and two electrons in the oxidation of a substrate to form NADH
The reactive part is its nicotinamide ring.
FADH2
An activated carrier of electrons for fuel oxidation
Flavin adenine dinucleotide (FAD)
accepts two protons and two electrons in the oxidation of a substrate to form FADH2
the reactive part is its isoalloxazine ring
NADPH
Activated carrier of electrons for reductive biosynthesis
In most biosynthesis, precursors are more oxidized than the products
ATP and reducing power are needed
ex. four electrons are needed to reduce a keto group to a methylene group
NADPH is the electron donor in most reductive biosynthesis
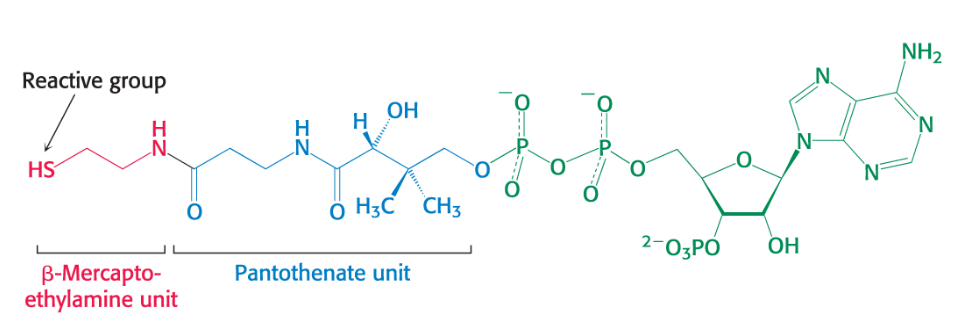
Coenzyme A
Activated Carrier of two carbon fragments
Carrier of acyl groups that is derived from vitamin B5 (pantothenate)
The reactive part is its terminal sulfhydryl group.
Acyl groups are linked to CoA by thioester bonds to form an acyl CoA.
Acetyl linked to CoA is called called acetyl CoA.
Why is the transfer of the acyl group exergonic?
The thioester is thermodynamically unstable
The ∆G°′ for the hydrolysis of acetyl CoA has a large negative value.
Electrons of the C=O bond cannot form resonance structures with the C—S bond that are as stable as those that they can form with the C—O bond
What does kinetic stability allow for?
Enzymatic control over the flow of energy
NADH, NADPH, and FADH2 react slowly with O2 in the absence of a catalyst.
ATP and acetyl CoA are hydrolyzed slowly in the absence of a catalyst.
What are they key reactions that are reitered throughout metabolism? (there are 6)
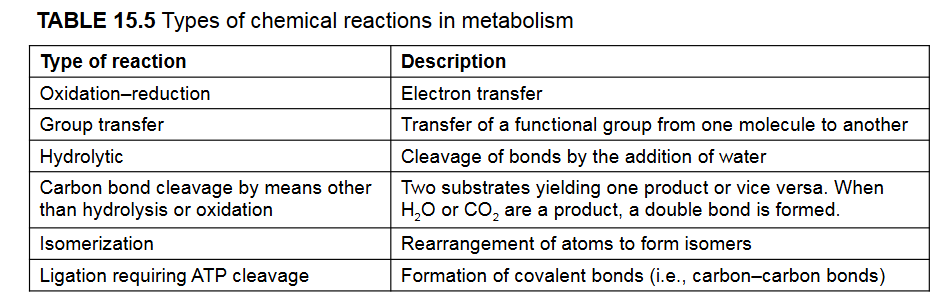
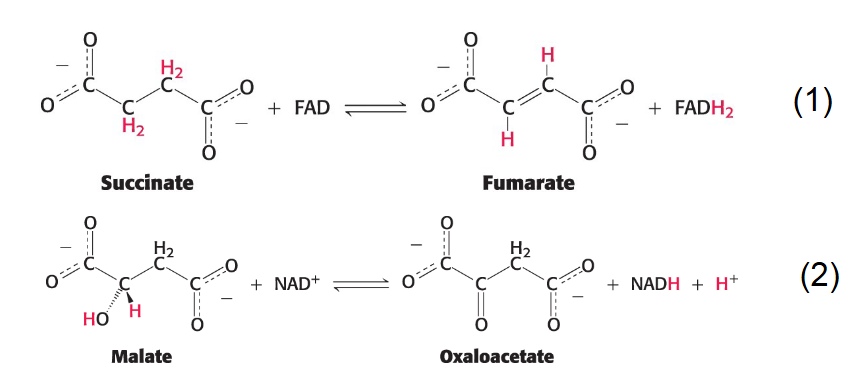
Oxidation-Reduction Reactions
Derives useful energy
ex. oxidation-reduction reactions of the citric acid cycle
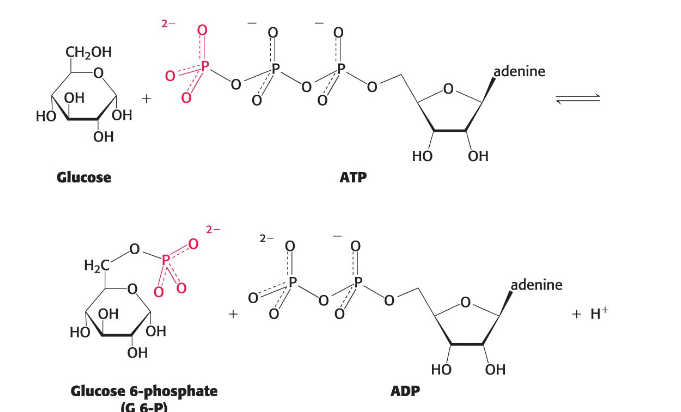
Group-Transfer reactions
Used to synthesize ATP and in signaling pathways among others
ex. phosphoryl group transfer
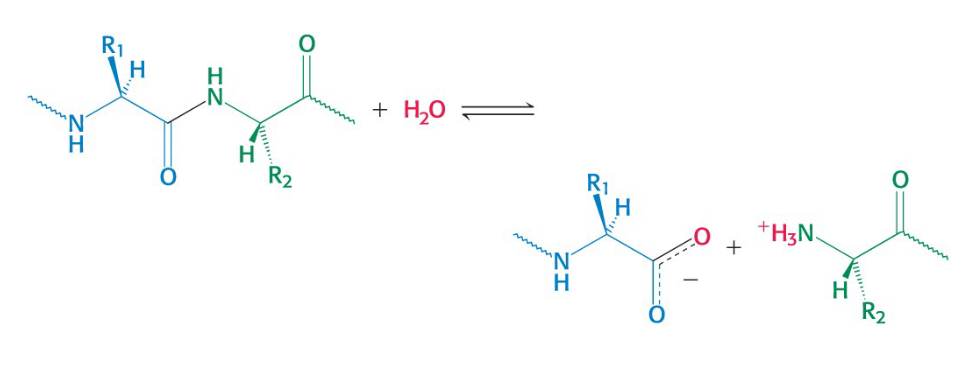
Hydrolytic Reactions
Hydrolysis cleaves bonds by the addition of water
commonly used to degrade large molecules
(if you see water, it’s very likely hydrolysis)
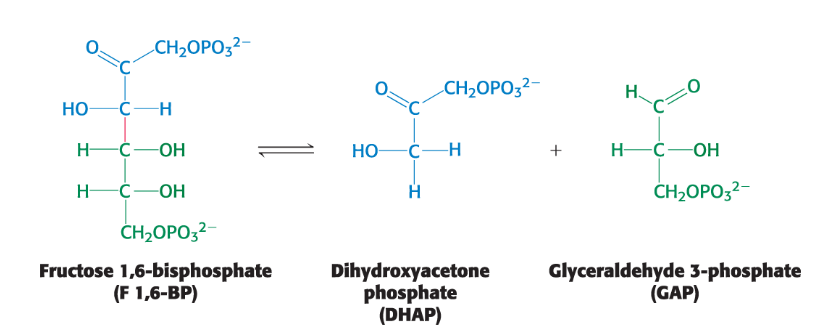
Carbon Bond Cleavage
Can occur by means other than hydrolysis and oxidation
ex. the conversion of the six-carbon molecule fructose 1,6-biphosphate into two 3-carbon fragments during glycolysis
Dehydration is an important subclass
ex. generation of phosphoenolpyruvate from 2-phosphoglycerate
Isomerization reactions
Rearranges atoms within a molecule
Typically to prepare the molecule for a subsequent reaction
example: the conversion of citrate to isocitrate
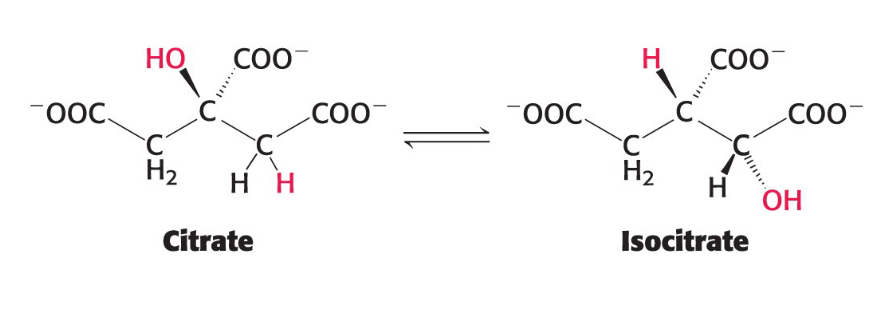
Ligation Reactions
Forms bonds using free energy from ATP hydrolysis
example: the formation of oxaloacetate from pyruvate and CO2
Pyruvate + CO2 + ATP + H2O → Oxaloacetate + ADP + Pi
3 principal ways metabolic processes are regulated
altering the amount of enzymes
The amount of a particular enzyme depends on both its rate of synthesis and its rate of degradation.
The level of many enzymes is adjusted by a change in the rate of transcription of the genes encoding them.
restricting the accessibility of substrates
Comparmentalization often segregates opposed restrictions
examples: fatty acid oxidation occurs in the mitochondrial matrix, while fatty acid synthesis occurs in the cytoplasm
Regulating the catalytic activity of enzymes internally or externally
Catalytic activity is regulated allosterically or by covalent modification.
Feedback inhibition is an example of allosteric regulation.
Concentrations of allosteric activators and inhibitors can be changed.
Reversible covalent modification can control catalytic rates of enzymes.
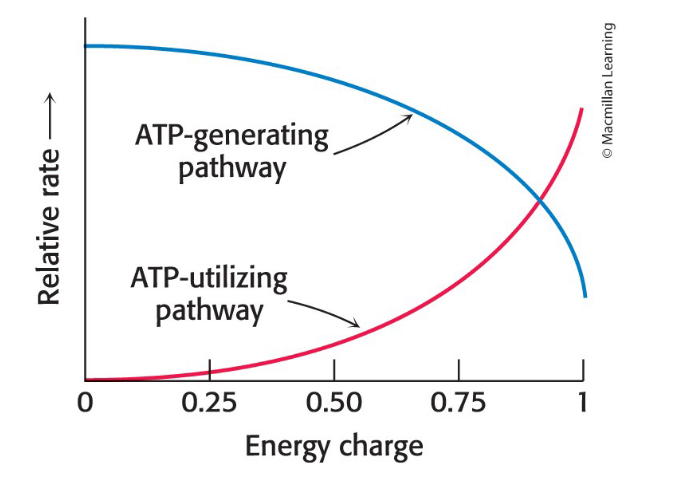
Energy Charge
Proportional to the mole fraction of ATP plus half the mole fraction of ADP
Ranges from 0 (all AMP) to 1 (all ATP)
Cells have to maintain energy charge between two values
High energy charge → ATP inhibits relative rates of a typical ATP-generating pathway and simulates the typical ATP-utilizing (anabolic) pathway