Principles of Chemistry D-E
1/18
Earn XP
Description and Tags
for section e (Chemical formulae, equations and calculations), it is more useful to do practice questions using the calculations than flashcards.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
How are the elements arranged in the periodic table?
in order of atomic number and in groups and periods
How many electrons can shells hold?
2,8,8
Groups
Columns
Periods
rows
Metal Propeties
Good conductor of electricity and basic oxide
Non metal properties
Poor conductor of electricity and acidic oxides (some are neutral)
Base
a chemical substance with a pH higher than 7 (alkaline)
Where are the metals in the periodic table?
On the left
Where are the non-metals in the periodic table?
on the right
Elements in the same group have..
the same number of outer electrons
Group 1 metals are reactive
as you go down the group
Group 7 elements are reactive
as you go up the group
The group number on the periodic table indictates
the number of electrons in the outer shell
Why are noble gases unreactive?
Because they have a full outer shell
How to calculate Mr
add up the atomic masses of all the atoms
Mol
the unit for the amount of a substance
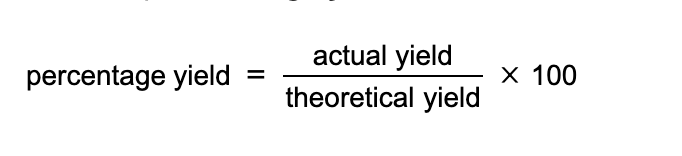
Percentage yield calculations
Molecular formula
the formula that shows the number and type of each atom in a molecule
Empirical formula
the simplest whole number ratio of each atom