Swine Infectious Diseases: Respiratory and diarrheal diseases
1/160
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
161 Terms
Organisms that cause atrophic rhinitis in pigs?
P. multocida, B. bronchiseptica, and H. parasuis
How do the organisms that cause atrophic rhinitis and cytomegalovirus differ in pathogenesis?
Porcine cytomegalovirus (CMV), which is the cause of inclusion
body rhinitis, does not cause nasal turbinate atrophy. However, it may damage the nasal mucosa, predisposing it to colonization with one of these bacterial agents.
What are predisposing factors that cause Atrophic Rhinits?
high ammonia levels (50–100 ppm) and dust (Hamilton et al., 1999), and genetics as well as infection by cytomegalovirus.
What toxins are produced by pasteurella and bordatella that lead to atrophic rhinitis?
P. multocida strains A and D produce Pasteurella multocida toxin (PMT), which causes progressive nasal turbinate atrophy, which disrupts G-protein and rho-dependent pathways and stimulates mitogenesis resulting in degenerative and hyperplastic changes, especially within the bony turbinates of the nasal cavity. B. bronchiseptica produces a heat-labile dermonecrotic toxin (DNT) which alone will produce a moderate self-limiting form of the disease in which damaged tissues may regenerate in time.
These toxins include DNT, which
impairs bone formation; adenylate cyclase toxin (ACT),
which is responsible for disruption of innate immune
function; tracheal cytotoxin (TCT), which interacts with
LPS and is responsible for impaired ciliary function
Which cause more severe atrophic rhinitis (or progressive atrophic rhinitis)?
-Bordatella alone
-Bordatella and pasteurella together
-Bordatella and hemophilus parasuis together
-Pasteurella and Hemophilus parasuis together?
Bordatella and Pasteurella
Which cause more severe atrophic rhinitis (or non-progressive atrophic rhinitis)?
-Bordatella alone
-Bordatella and pasteurella together
-Bordatella and hemophilus parasuis together
-Pasteurella and Hemophilus parasuis together?
Recently, the term ‘nonprogressive atrophic rhinitis’
(NPAR) has been applied to the form caused by B. bronchiseptica alone, and the term ‘progressive atrophic rhinitis’
(PAR) to P. multocida alone and combined infections and B. bronchiseptica
Transmission of bordatella
B. bronchiseptica is spread from pig to pig by aerosol
droplets, which probably first occurs with snout-to-snout
contact between a sow and a newborn piglet,. Often from carrier pigs in a new herd. B. bronchiseptica colonizes the ciliated epithelial cells in the nasal epithelium.
Which immunoglobulins are required to clear bordatella from the immune system?
IgG and IgA are both required for complete clearance of the respiratory tract of B. bronchiseptica.
What conditions are required for P. multocida to colonize?
In order for P. multocida to colonize, decreased ciliary
function or increased mucous is typically required.
Clinical signs of bordatella bronchiseptica based on age?
The clinical signs of pure B. bronchiseptica infection (NPAR) generally appear in nursery pigs less than 4 weeks of age and consist of sneezing, snuffling, and a mucopurulent nasal discharge. In older pigs, these signs are mild or nonexistent.
Clinical signs of P. multocida based on age?
The clinical signs of P. multocida (PAR) typically
begin at 1–3 months of age and consist of sneezing and
snuffling, which progresses to more violent sneezing
with mucopurulent nasal discharge.
Most characteristic clinical signs of P. multocida?
The most characteristic clinical sign is the dorsal and/or
lateral deviation of the snout as the pig grows. This is
caused by abnormal bone growth due to unequal nasal
turbinate atrophy. Brachygnathia superior is the most
common form seen and is due to slower bone growth in
the upper jaw which gives it an upturned appearance.
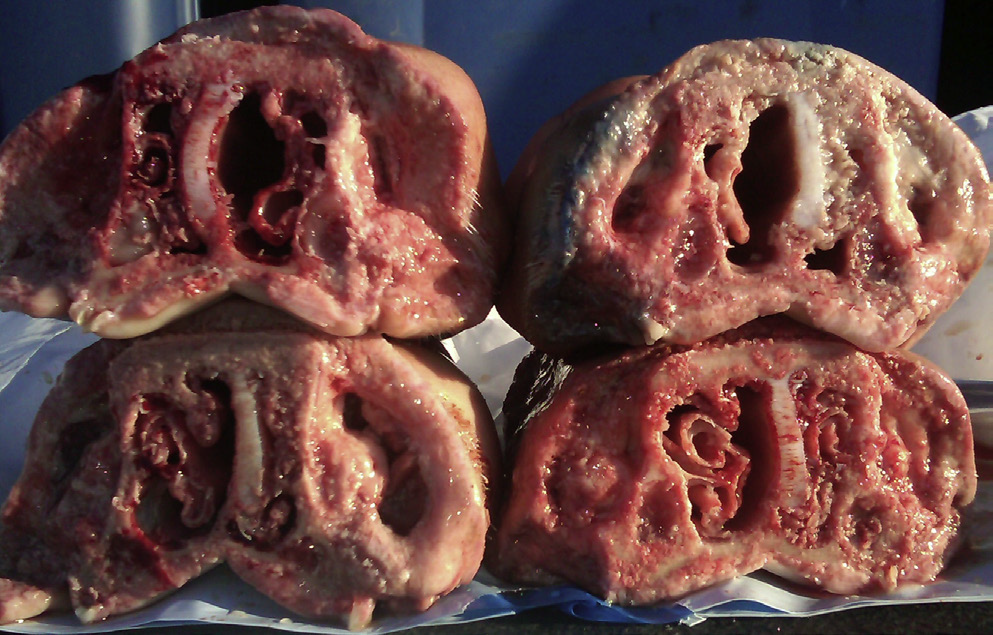
What condition is this photo showing?
Atrophic rhinitis
Can atrophic rhinitis be cured?
Once lesions are present, there is no
cure. AR is a disease best prevented.
What is the treatment and prevention plan for NPAR and PAR?
A treatment plan for NPAR and PAR should include a combination of environmental and husbandry improvements followed by a vaccination and antibiotic program tailored to the facility.
Vaccines are generally given to the sow prefarrowing, as improved colostral immunity is considered more important than piglet vaccination
Which scroll is most commonly affected by atrophic rhinitis?
atrophy of the turbinates (most severe in the ventral scroll of the ventral turbinate)
What anatomical location is used by pathologists to cut to diagnose atrophic rhinitis?
turbinate atrophy is subjectively measured
at necropsy by visual scoring of a section at the
level of the second premolar
What other organ systems can be affected by Bordatella or P. multocida?
The toxin produced by severe infections of toxigenic strains of P. multocida will induce liver and kidney lesions as well as damage nasal turbinates.
What capsular serotypes are found in swine with P. multocida, which are most likely to cause lung disease and which are most likely to cause the most severe disease?
Capsular serotypes A, B, and D have been reported in swine, A being the most common in pneumonic lungs and B causing the most severe disease (septicemic)
Is P. multocida naturally found in the nasal cavity or always pathogenic when found?
P. multocida is a common inhabitant of the upper respiratory tract of swine.
Which infectious diseases cause thumping in pigs?
Dzs that cause thumping:
1. P. multocida
2. A. pleuropneumonia
3. M. hyopneumonia
4. Metastrongylus
What diseases are the acute and chronic form of P. multocida similar to?
The acute form is clinically similar to pleuropneumonia
(APP) without the frequency of sudden death; the
chronic form is similar to mycoplasmal pneumonia of
swine (MPS)
Is P. multocida usually a secondary or a primary pathogen?
secondary
What are common histopathologic findings of P. multocida?
Gross findings in the lungs are usually
confined to the cranioventral aspects of the lobes and
include red to gray areas of consolidation, frothy exudate
in the trachea, suppurative pleuritis and pericarditis,
pleural adhesions, and pulmonary abscesses
What is the etiology of Actinobacillus pleuropneumonia and what protein is required for its growth?
This bacterium is a gram-negative encapsulated
coccobacillary rod, which requires nicotinamide adenine
dinucleotide (NAD or factor V) for growth.
What are the three most important virulence factors of Actinobacillus pleuropneumonia?
Extracellular hemolytic toxins ApxI, ApxII, and ApxIII
are some of the more important virulence factors
Transmission of actinobacillus
Transmission is primarily by snout to snout and by aerosol
How quickly does death occur after innoculation?
Inoculation of pigs may result in death in as little as 3 h
Where does APP bind in the body?
APP binds squamous cells of the tonsil
followed by type I pneumocytes of the lower respiratory
tract
What is the pathogenesis of APP?
Tissue damage is primarily caused by the host immune response and from lytic Factors released from these cells when they are killed by Apx toxins released from APP, whereas death is primarily due to endotoxic shock from APP LPS
Common clinical presentation of APP
The peracute form is characterized by
rapid development of fever to 41.7°C, anorexia, and
depression.
the skin becomes cyanotic beginning at the extremities
Animals may be found dead with no prior signs
(pleuritis, abortion, endocarditis, arthritis, abscesses).
Dyspnea
Control of APP
treatment not rewards, maintaining APP herds essential
APP histopath findings?
Necropsy The gross findings in pigs with APP
are dependent upon time course and include fibrinous
pleuritis, pulmonary edema, and the presence of bloody
froth or clotted fibrin plugs in the trachea and bronchi.
The lungs contain bilateral lesions that are dark red and
firm with a predominance of lesions in the dorsal aspects
of the caudal lobes, and there may be a bloody nasal
discharge
What is the etiology of actinobacillus suis?
Actinobacillus suis is a gram-negative
bacterium in the Pasteurellaceae family.
Where does Actinobacillus suis colonize and is it common in pig herds?
A. suis colonizes the
upper respiratory tract with many herds infected, all of
which do not always show clinical signs.De
Describe the three forms of Actinobacillus suis based on age?
The first form is a septicemia
affecting suckling and recently weaned piglets
where the only signs may be that animals are found dead.
The second form is respiratory disease seen in growers
and finishers with clinical signs including cough and
fever, but they may also be found dead.
3. The third form
is an acute septicemia that affects adults with lethargy,
anorexia, fever, rhomboid skin lesions, and abortion in
pregnant sows.
Which other disease in pigs are findings similar for A. suis on necropsy?
Lesions include erysipelas-like lesions,
What is the agent that causes Mycoplasmal Pneumonia: Enzootic Pneumonia, Mycoplasmal Pneumonia of Swine?
Mycoplasma hyopneumoniae
Where does mycoplasma hypneumonia colonize?
M. hyopneumoniae is a common pathogen that colonizes the ciliated epithelium of the porcine respiratory tract.
What is the etiology of mycoplasma?
Mycoplasmas are small (0.2–0.3 μm), lack
a cell wall, and are nonmotile, fastidious, gram-negative
facultative anaerobes.
Is M. hyopneumonia is more likely enzootic or epizootic in pigs?
Enzootic disease is what is most commonly seen.
What pathogen is the most common cause of chronic pneumonia
mycoplasma hyopneumonia
At what age is M. hyopneumonia generally clinically apparent in pigs?
generally clinical signs are not
obvious until pigs are 3–6 months of age.
What two pathogens does M. hyopneumonia predispose pigs to?
It plays an important role in PRDC when concurrent infection with PRRSV or PCV2 occurs
Are mycoplasma’s easy to culture? What diagnostic modalities are used to identify mycoplasma?
Culture is not usually feasible since mycoplasmas and
M. hyopneumoniae, in particular, are difficult to isolate
and grow. IFA or IHC and PCR have all been used in
diagnosis. ELISAs are also currently
in use to diagnose M. hyopneumoniae
How do you treat Mycoplasma Hyopneumonia?
Experimental evidence has shown
that doxycycline has greater in vitro activity than oxytetracycline
against M. hyopneumoniae, A. pleuropneumoniae,
and P. multocida
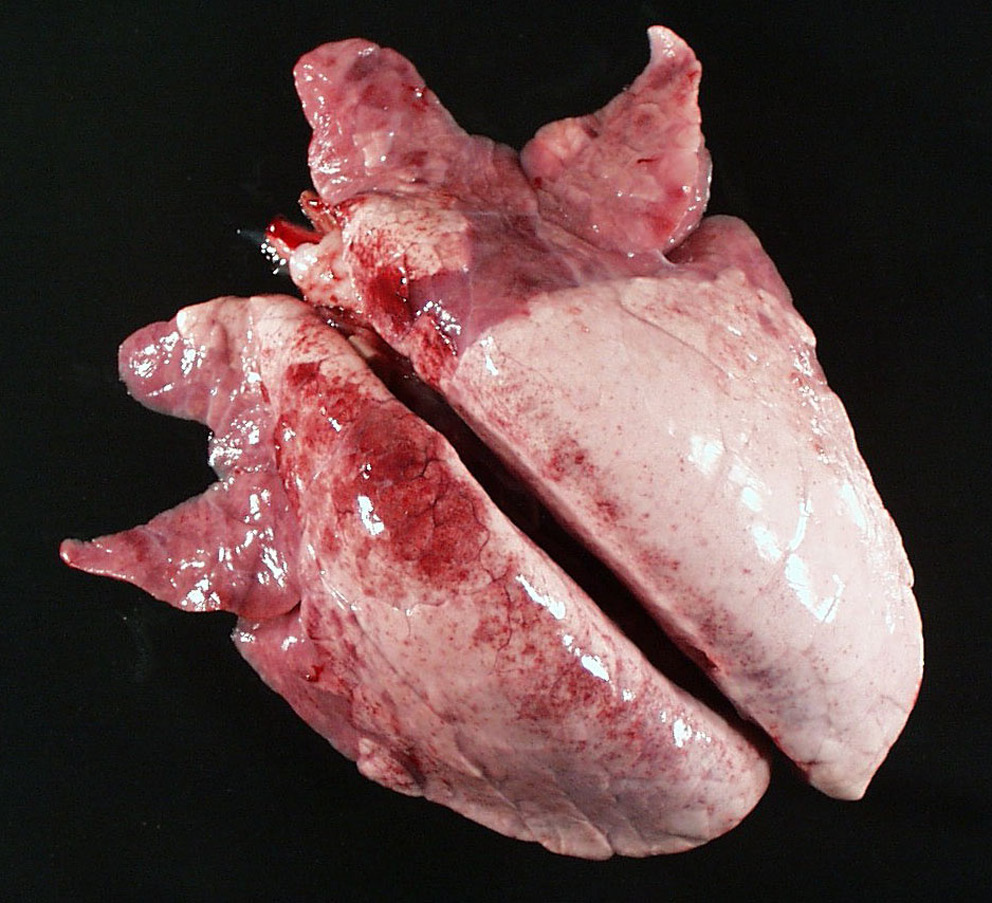
What is likely the pathogen causing these gross necropsy lesions?
Mycoplasma Hyopneumonia
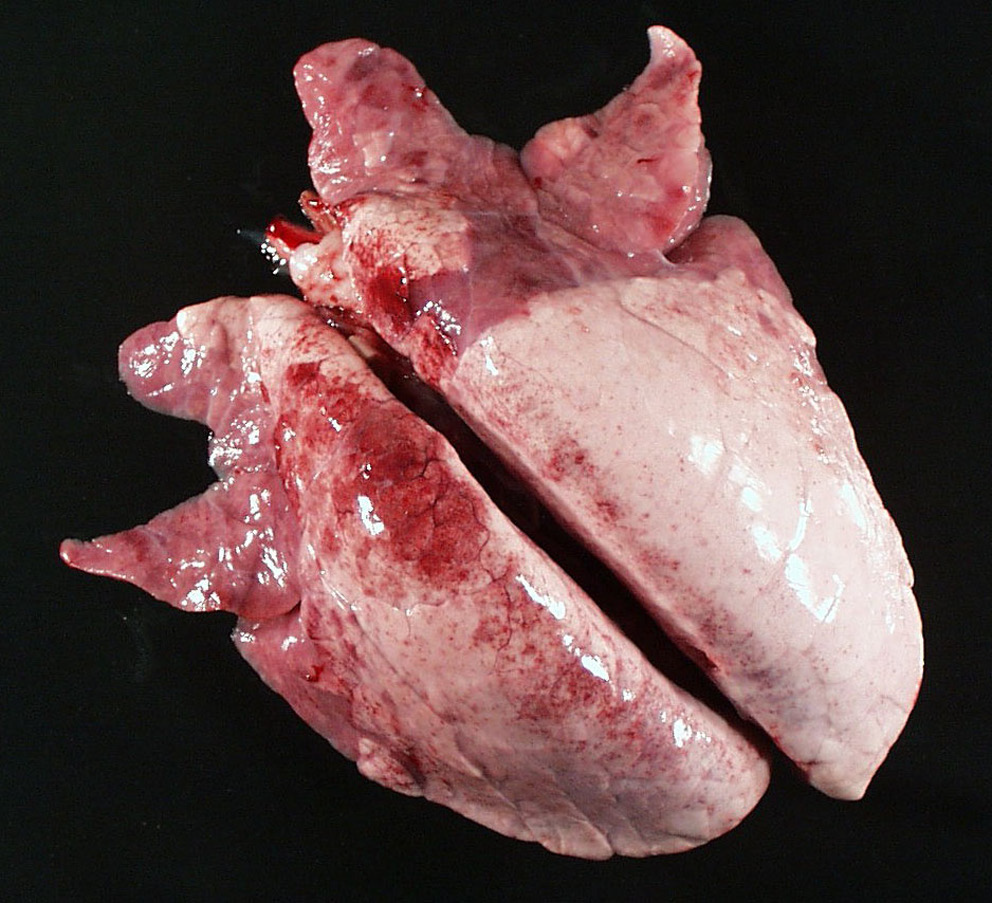
Regarding control of mycoplasma in swine herds, are swine immune after having M. hyopneumonia? Is there a vaccine? Does it work?
Yes swine are immune after infection and there is a vaccine that appears to work well for alleviating disease but not prevention of infection.
What are the classic pathology findings for M. hyopneumonia?
In acute disease, the lungs fail to collapse
and there is edema of the lungs. The lungs contain pale
gray or dark-red foci of consolidation that are most
commonly found in the apical lobes and the cranioventral
aspects of the middle, accessory, and caudal lobes. Additionally, there may be catarrhal exudate.
Which mycoplasma species is the easiest to isolate that affects swine?
Mycoplasma hyorhinis is probably the easiest of the porcine mycoplasmas to isolate, is a common contaminant of cell culture lines, and is ubiquitous in the swine population.
What is the common name for Mycoplasma hyorhinis in pigs?
Mycoplasmal Polyserositis and Arthritis
Which mycoplasma species of swine is the most likely to infect via cell culture lines?
M. hyorhinis
Is it common for M. hyorhinis to be found in the respiratory tract of swine without any clinical signs?
This organism is harboured in the respiratory tract of carrier swine, often without clinical disease. The organism adheres to cilia similar
to M. hyopneumoniae and is considered normal flora of
the respiratory tract.
What age groups are most predominantly clinically affected by M. hyorhinis? What are the clinical signs?
It causes polyserositis and polyarthritis in pigs less than 8 weeks old. In older pigs (3–6 months old), only arthritis is caused. The age group most commonly affected is 3–10 weeks of age. agent. The acute signs are lethargy, anorexia, labored respirations, arched back with tucked-up abdomen, lameness, and slight fever and swollen joints. These signs abate in about 2 weeks except that the lameness with swollen joints may persist for several months. Experimental
Common necropsy findings for M. hyorhinis?
Acute lesions include serofibrinous or fibrinopurulent pleuritis, pericarditis, and peritonitis, as well as serofibrinous arthritis with increased synovial fluid and swollen reddish yellow synovial membranes.
Inclusion body rhinitis is caused by what type of virus? What are a hallmark cytology finding for Inclusion Body Rhinitis?
Inclusion body rhinitis (IBR) is caused by porcine cytomegalovirus (PCMV) and is found through out the world. The causative agent is a member of the subfamily Betaherpesvirinae, genus Proboscivirus that produces cytomegaly with hallmark basophilic intranuclear inclusions in cytomegalic cells of nasal mucosa.
DNA enveloped, family herpesviridae
How is inclusion body rhinitis spread?
The virus can also be transmitted transplacentally.
Dissemination of the agent most commonly occurs
via nasal secretions and urine.
After what age is inclusion body rhinitis subclinical?
This disease is usually subclinical in pigs more than 3 weeks of age.
What are the common clinical presentation of Inclusion body disease in young pigs, under 3 weeks of age?
The clinical sequelae typically associated with this disease include unexpected fetal and piglet death, runting, rhinitis, conjunctival discharge, pneumonia, sometimes neurological signs, and poor weight gain in young pigs
Common necropsy findings of inclusion body disease?
The clinical sequelae typically associated with this disease include
unexpected fetal and piglet death, runting, rhinitis, conjunctival
discharge, pneumonia, sometimes neurological
signs, and poor weight gain in young pigs
What letter type of influenza causes most common swine influenza?
Influenza A, Influenza A viruses belong to the family of RNA viruses, Orthomyxoviridae, Enveloped
Transmission of influenza in a swine herd?
Swine influenza typically appears as a result of new animals entering the herd. Outbreaks rapidly spread through all animals within a group.
The primary route of transmission is via direct contact.
The swine influenza viruses (SIVs) have a very wide
host range.
The virus enters via the respiratory
epithelium.
Clinical presentation of influenza in swine
Animals appear very ill with anorexia, labored open-mouthed breathing, and a strong reluctance to move.
Clinical signs of influenza in swine
Clinical Signs/Diagnosis: Animals appear very ill
with anorexia, labored open-mouthed breathing, and a
strong reluctance to move. The animals have fever, rhinitis,
and nasal discharge, and during recovery, will cough.
Despite the apparently severe clinical signs, the animals
typically recover rapidly within 5–7 days of developing
clinical signs. Morbidity is nearly 100% with less than 1% mortality
Treatment of influenza in swine
amantadine has been shown to reduce the febrile response and the shedding of virus in experimentally infected pigs.
Gross findings on necropsy for influenza in pigs?
There is fibrinous to mucopurulent exudate in nasal passages, trachea, bronchi, and bronchioles, and sharply demarcated dark-red to purple firm foci of consolidation in apical and cardiac lobes of the lung along with interlobular edema.
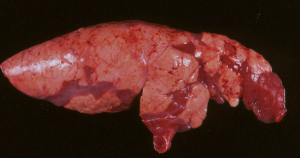
What are the common species that cause Verminous Pneumonia (Verminous Bronchitis)?
Metastrongylus spp. include one or more of M. salmi,
M. pudendotectus, or M. elongates apri, with the latter
being the most common.
Ascaris suum
Which parasites have a direct cycle vs. indirect cycle for verminous pneumonia?
Metastrongylus has indirect lifecycle (earthworm), Ascaris has a direct lifecycle (still risk of transmission in doors)
Prepatent periods for metastrongylus and ascaris?
Prepatent period 28 days for metastrongylus
40 - 53 days for Ascaris
Pathogensis of Metastrongylus elongatus?
M. elongatus larvae migrate through
the lung parenchyma, causing alveolar hemorrhage fol
lowed by inflammation and consolidation of the lungs. Eggs are coughed, swallowed and excreted in feces.
Pathogenesis of Ascaris suum?
Migrating A. suum create liver lesions (white spots), which are
seen grossly as white spots that peak at about 1 week
post infection. The pathogenesis of the lung lesions is similar to that of M. elongatus; however, the larvae are coughed up and
then swallowed and mature into adults in the small
intestine.
Clinical signs of verminous pneumonia?
clinical signs consist of dyspnea and decreased weight gain.
What is best for sanitation for verminous pneumonia?
Neopredisan (p-chloro-m-cresol)
disinfectant has been shown to be a very efficacious ovicide
and larvicide for A. suum
Risk factors for diarrhoea in young pigs
Young swine commonly develop diarrhea associated
with shipping stress, changes in diet, primary or mixed
infection with a variety of enteric pathogens, or the perioperative
use of antibiotics that may upset the balance
of normal gut microbiota.
Cause of swine dysentery?
Brachyspira hyodysenteriae, a gram-negative anaerobic spirochete
What is the hallmark clinical sign and what age is most affected by swine dysentary?
severe mucohemorrhagic diarrhea of pigs of postweaning age
What types of diets predispose and are protective against infection by B. hyodysentariae?
diets rich in rapidly fermentable carbohydrates may
exacerbate clinical signs whereas highly digestible diets,
or those high in inulin are protective against disease
Transmission of B. hyodysenteriae?
Fecal oral
Can transmit after clinical signs stop.
Pathogenesis of B. hyodysenteriae
These organisms do not invade the gut wall below
the lamina propria. The organism produces a hemolysin
that is cytotoxic and an endotoxin. Diarrhea caused by
B. hyodysenteriae is the result of colonic malabsorption
from failure of colonic epithelial cells to transport sodium
and chloride from the lumen to the blood (Argenzio
et al., 1980; Schmall et al., 1983). The mechanism of diarrhea
is therefore very different from that of Salmonella,
Shigella, and E. coli
Clinical signs of Swine dysentery?
severe diarrhea and fever with
accompanying dehydration, weight loss, and weakness
develop over several days. Diarrhea of acute onset is
usually watery with large amounts of mucus
Diagnosis of swine dysentery?
Culture, Warthin–Starry positive spirochetes in colonic crypts, PCR
Control methods for Swine Dysentery?
Swine dysentery is usually
introduced to a facility by the purchase of an asymptomatic
carrier pig. Wild rodents are also reservoirs. Pigs
should be purchased from herds SPF for B. hyodysenteriae
Where are gross lesions confined to in the pig?
Large Bowel
What causes Porcine Intestinal/Colonic Spirochetosis?
Brachyspira pilosicoli
What ages does B. pilosicoli affect?
intestinal spirochete that causes porcine colonic spirochetosis, a nonfatal diarrheal disease that affects pigs during the growing and finishing stages
Transmission dynamics of B. pilosicoli? Is it zoonotic?
B. pilosicoli can be found in contaminated water and colonizes chickens, wild ducks, and immunocompromised humans. Transmission is fecal–oral
What type of diet increases the risk of B. pilosicoli?
pelleted diets increase the risk of colonization
Clinical signs of B. pilosicoli?
Signs include loose stool in
finishers, but younger animals often have watery green
to brown mucoid diarrhea with flecks of blood (Duhamel
et al., 1998). Concurrent diseases that may lead to exacerbation
include swine dysentery, salmonellosis, proliferative
enteropathy, or PCV2 infection
Where are gross lesions primarily in B. pilosicoli?
Gross lesions are limited to the cecum and
colon. Organisms may also be found
within goblet cells
Cause of Proliferative enteropathy?
Lawsonia intracellularis
Are Lawsonia itnracellularis difficult to culture?
Yes, only grow on tissue culture
Other names for Lawsonia intracellularis in pigs and what is the hallmark necropsy finding?
the excessively proliferative lesions found
at necropsy in the terminal ileum, proliferative enteropathy
(PE) of the pig has historically been referred to
as porcine intestinal adenomatosis, terminal or regional
ileitis, intestinal adenoma, and porcine proliferative
ileitis.
Transmission dynamics for Lawsonia Intracellularis in pigs?
Lawsonia is shed in feces, and transmission is by fecal–oral contact. In endemic areas, 15–30% of the herds are estimated to be affected with a 5–20% infection rate within a herd. There is risk of environmental contamination, as L. intracellulare can remain viable in feces for at least 2 weeks. L. intracellulare has also been reported in wild pigs which may also serve as a source of infection
Pathogenesis of lawsonia
The infected crypt cells fail to mature and are not shed, so the crypts become elongated and tortuous resulting in decreased nutrient absorption.
Clinical signs and what age groups are affected?
Clinical disease attributed to PE is most often observed in postweaned pigs between 6 and 20 weeks of age. Clinical signs range from none to marked dullness, anorexia, and diarrhea. Diarrhea is typically moderate with loose to watery stools of normal color. More chronic form associated with passage of black tarry feces.
Treatment of Proliferative enteritis?
Tylosin phosphate can be effective for prevention and for
treatment of PE. Often self-limiting requiring supportive care in severe cases.
What are the necropsy findings for proliferative enteritis?
The gross lesions of PE are found in the
ileum, cecum, and the most proximal one-third of the
spiral colon and consist of a markedly thickened gut
wall and mucosa containing multiple transverse or longitudinal fold
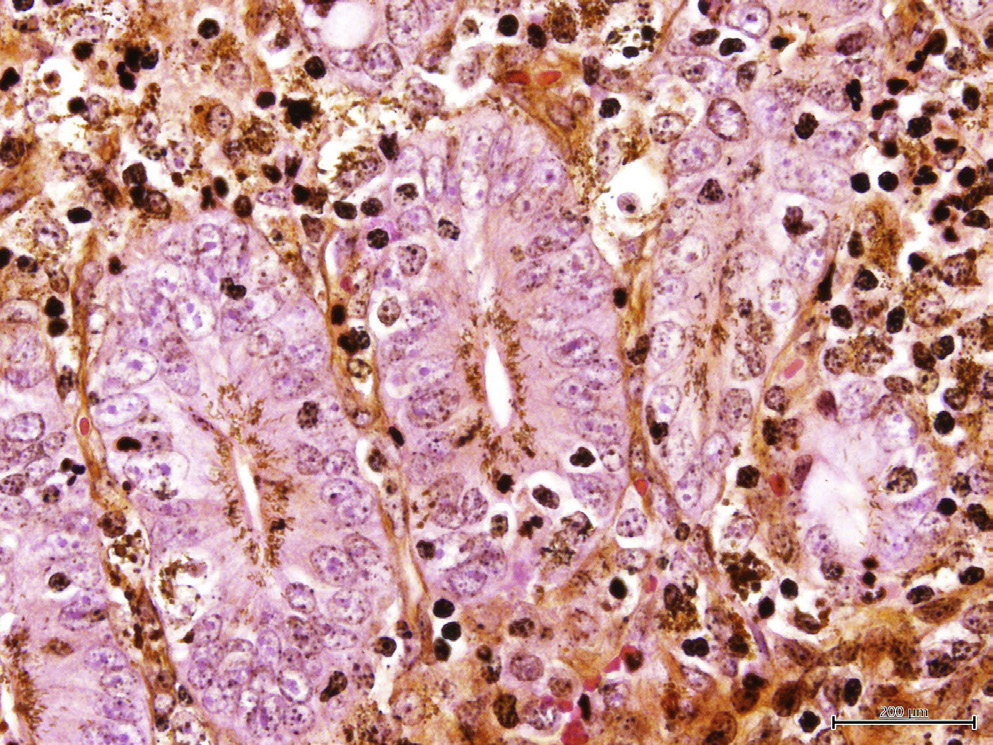
What is this?
Lawsonia intracellularis in the apical portion of the gut
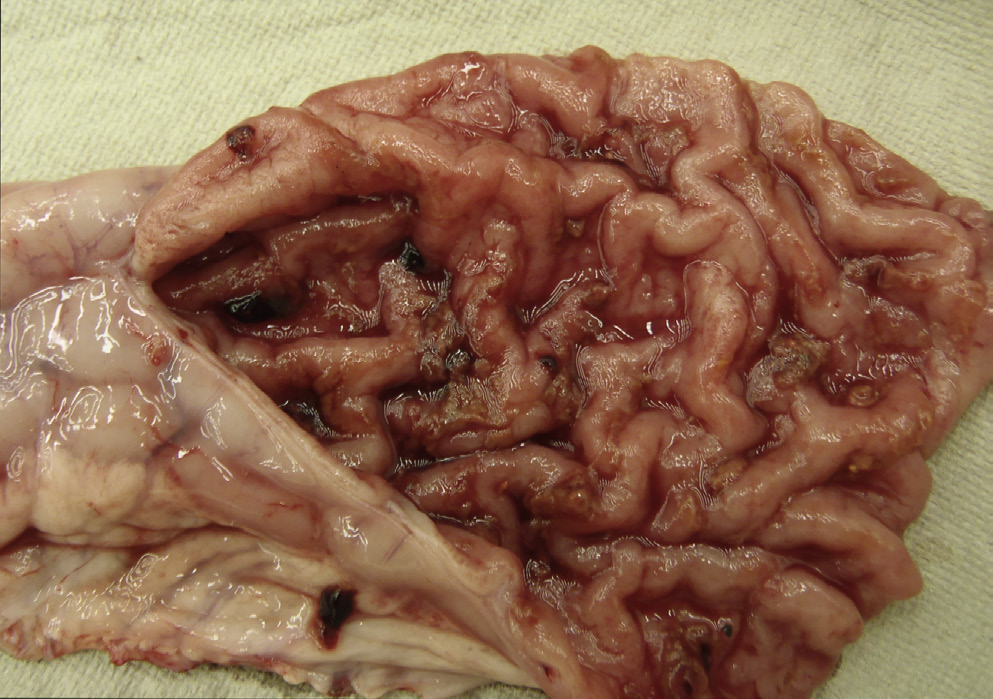
10 week old pig presents with loose watery diarrhea?
Lawsonia
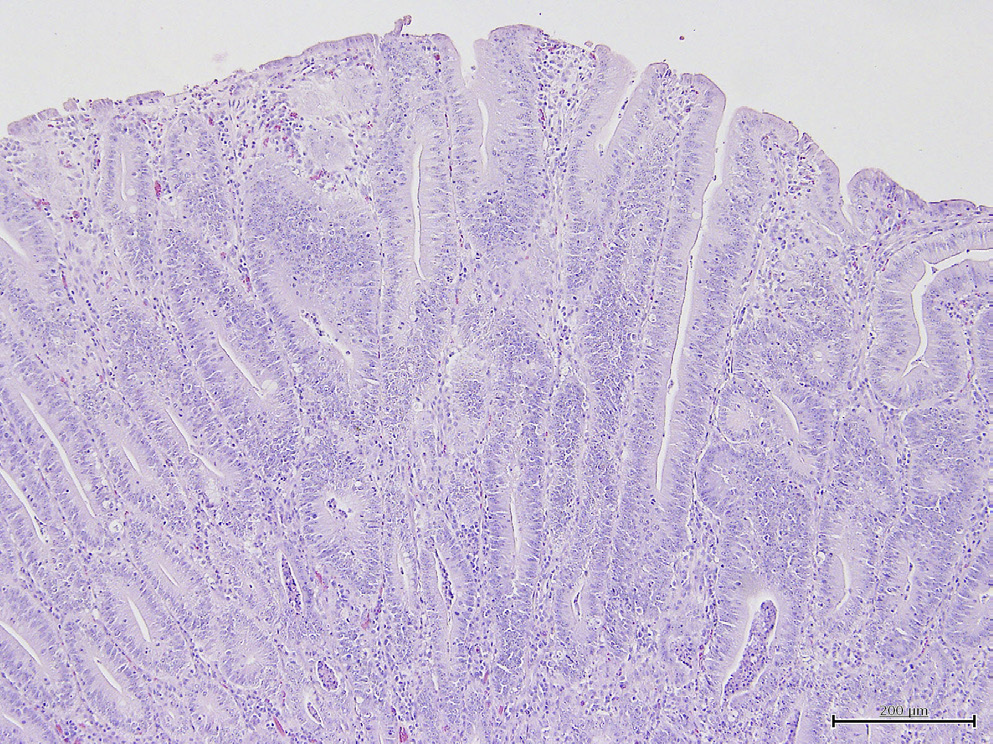
Pigs necropsied after dieing from dehydration associated diarrhea. Path photos show elongated villi.
Lawsonia