science test 2 - 9th grade
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Pure substance vs Mixture
pure substance is matter that always has exactly the same composition and a mixture is a combination of two or more elements and is not chemically bonded.
Element vs Compound
an element is a substance that cannot be broken down into simpler substances. A compound is a substance that is made from two or more simpler substances and can be broken down into these simpler substances.
Heterogeneous vs Homogeneous
Homogeneous is when all particles in the mixture look the same throughout. Heterogeneous is when particles look distinctly different
What is similar about compounds and mixtures? What is different about compounds and mixtures?
similar: both made of more than one type of element. different: compounds are bonded together and they have a specific ratio of elements needed while mixtures don’t and are not bonded together.
give examples of heterogeneous and homogeneous
Heterogeneous: soup
Homogeneous: flat soda
Define physical and chemical properties. What are examples of each?
Physical properties: characteristics you can observe/test (color, density, volume, boiling/melting point)
Chemical properties: a property’s ability to undergo changes that change the substance (rust, flammability, ability to react to acid)
What is different about chemical and physical changes?
Physical: Composition of substance does not change
Chemical: Composition changes, creating a new substance
What is similar about chemical and physical changes?
Properties/characteristics of the substances change (physical: appearance & chemical: all properties)
What is an example of a chemical change and a physical change?
Chemical: wood burning or metal rusting
Physical: Ice melting
Explain the Kinetic Theory of Matter.
Particles:
Solids:
Liquids:
Gases:
Temperature increase
Temperature decrease:
Particles: everything is made of particles, they’re constantly in motion
Solids: particles close together in a defined shape, with little movement
Liquids: we have little space between particles, moving farther and faster than solids.
Gases: lots of space between particles, move very fast, no definite shape or volume
Temperature Increased: particles move faster
Temperature Decreased: particles move slower
How is “Gas Produced” from the signs of chemical change different from the phase changes of Solid → Gas or Liquid → Gas?
A new substance is being created when gas is produced from a chemical change (like in baking soda and vinegar) but when it's changing states it isn’t permanent and no new substance is being created, heat is necessary not mixing with another substance (boiling water)
What unit does temperature need to be in for Charles’s Law and Gay-Lussac’s Law problems?
Kalvin
Using the Kinetic Theory of Matter, describe what happens during a phase change.
Freezing: the particles in a substance, as they become cooler and cooler, start to slow down and once they hit 0 degrees celsius set into a shape and move less freely. They then are a solid and the particles only move and shake around in place.
Differentiate between suspensions, solutions, and colloids, and give examples of each. Which fit into the heterogeneous category, and which fit into the homogeneous category?
Suspension: A heterogeneous mixture that separates into layer over time: muddy water
Colloid: contains some particles that are intermediate in size between the small particles in a solution and the larger particles in a suspension: milk
Solution: When substances dissolve and form a homogeneous mixture, the mixture that is formed is a solution: salt water
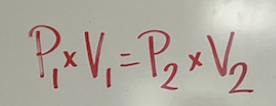
which law is this?
Boyle’s gas law
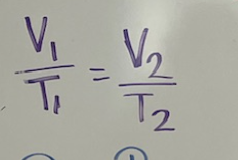
which law is this?
Charles’ gas law
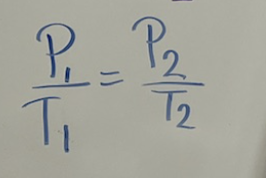
which law is this?
Gay-Lussac’s law
What are the 3 common names of the phase changes (from one state of matter to another) and which states of matter are involved in each?
Freezing, melting, and boiling. Solid, liquid, gas
Identify the relationships between pressure, temperature, volume, and number of gas particles (Be able to explain and describe the different gas laws).
Pressure and Temperature: if temperature increases then pressure will increase (gay-lussac’s law)
Temperature and Volume: if temperature goes up then volume will increase (charles’ law)
Volume and Pressure: if volume goes up then pressure goes down (boyle's law)
Identify the 5 signs of a chemical change.
Change in color, temperature, taste/smell, gas produced, precipitate formed (new solid that doesn’t mix in well - like the milk and coke) how lucky reprise