Tests for FINAL
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
77 Terms
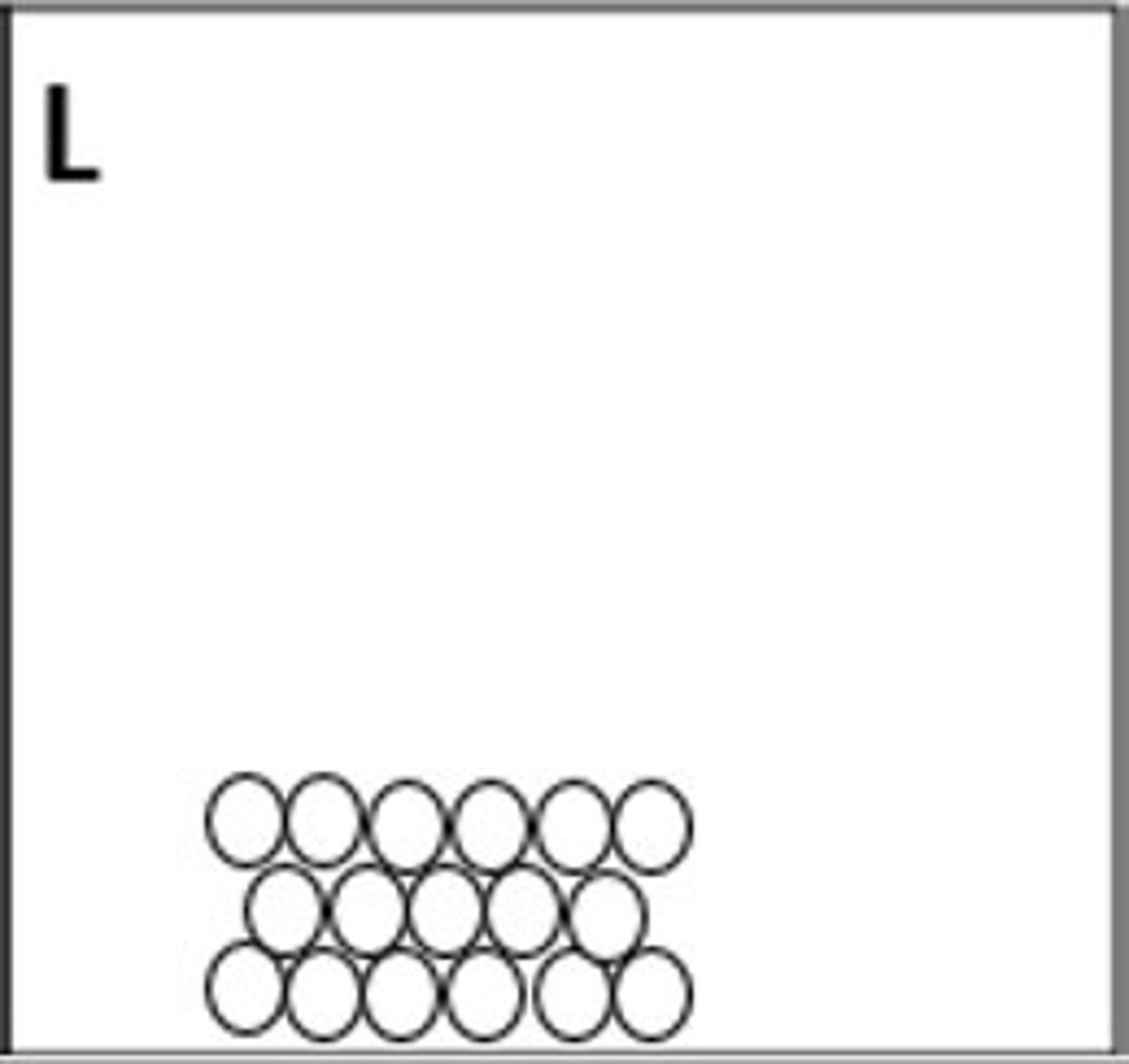
solid (B)
Which representation best illustrates a solid element?
Molecular compound
A substance is composed of one sulfur atom and two oxygen atoms chemically joined (bonded) together. What term best describes this substance?
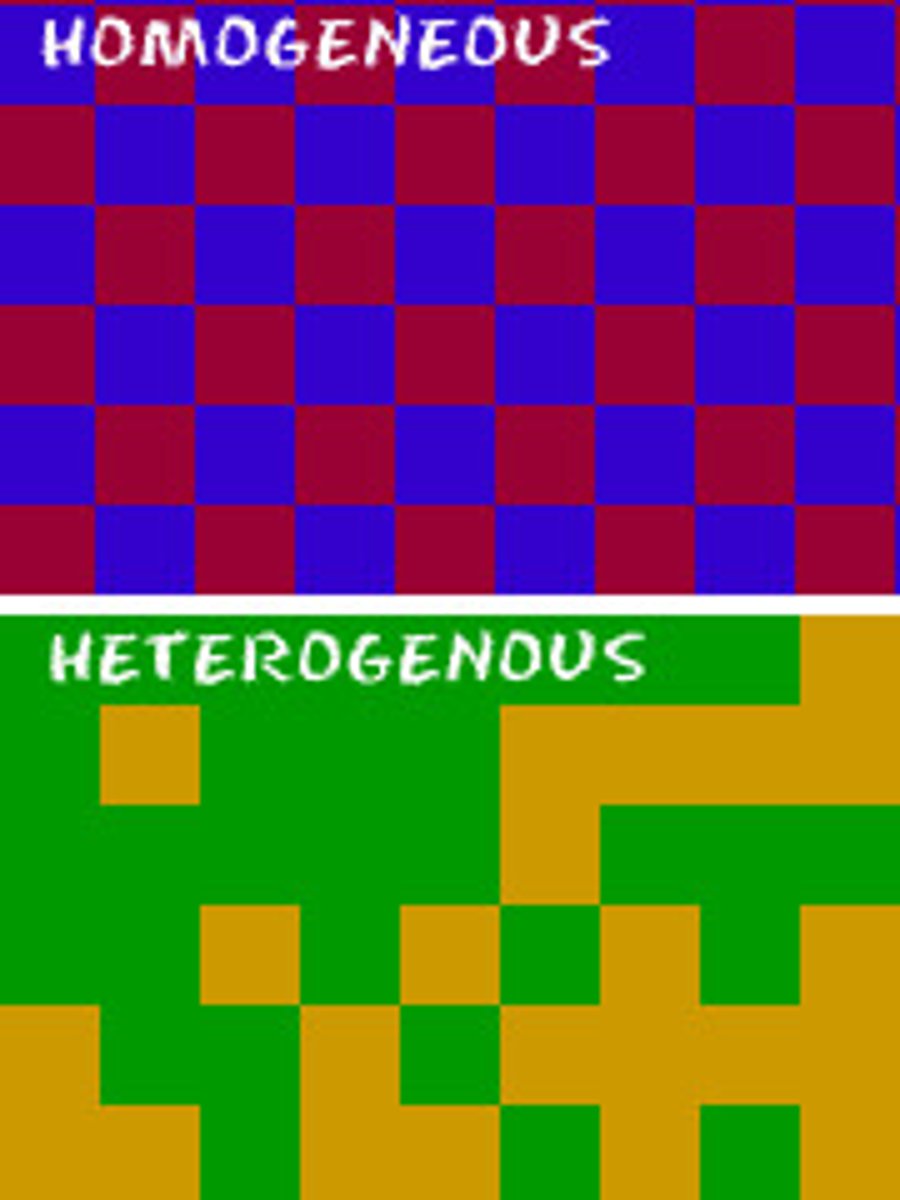
Heterogeneous mixture of an element and a compound
Which description is represented in the illustration?
Electrolysis of water into hydrogen gas and oxygen gas
Which would be a chemical change?
12.20 cm
What is the appropriate measure of the length of the rectangle?
micro
What would be the appropriate prefix to use for the measurement of the mass of argon in 1 mL of air. 1 mL of air contain about 0.00004 g of argon
6.05x10-8 m
Convert 60.5 nm to meters.
B. 29 C
Convert 85 oF to Celsius temperature.
D. 45,600 m
Convert 4.56x104 m into proper decimal notation.
E. 2.3 cm3
How many cubic centimeters (cm3) in 2300 cubic millimeters (mm3)?
D. 12.4 kg
Gold has a density of 19.3 g/mL. A standard bar of gold has a volume of 642 mL. What is the mass (in kg) of a standard bar of gold?
C. 22.99 g
A 58.44 g sample of sodium chloride contains 35.45 g of chlorine. What mass of sodium is present?
C. Rutherford's Gold Foil
13. Which experiment's key finding was the discovery of the proton?
NaBr
14. Which compound was made from an alkali metal and a halogen?
C. H2S
15. Knowing water is H2O, what is the expected formula for a compound containing hydrogen (H) and sulfur (S)?
15
16. How many protons in a phosphorous (P) atom?
B. An ion is an atom that has gained or lost electrons.
17. Explain how atoms turn into ions of the same element?
Na
18. Which element would be expected to have an ion with a +1 charge?
10
19. How many electrons in a Mg2+ ion?
D. 16.5 amu
What would be the average atomic mass of a hypothetical element if the element has only two isotopes? Mass AMU= 1= 15 2= 17
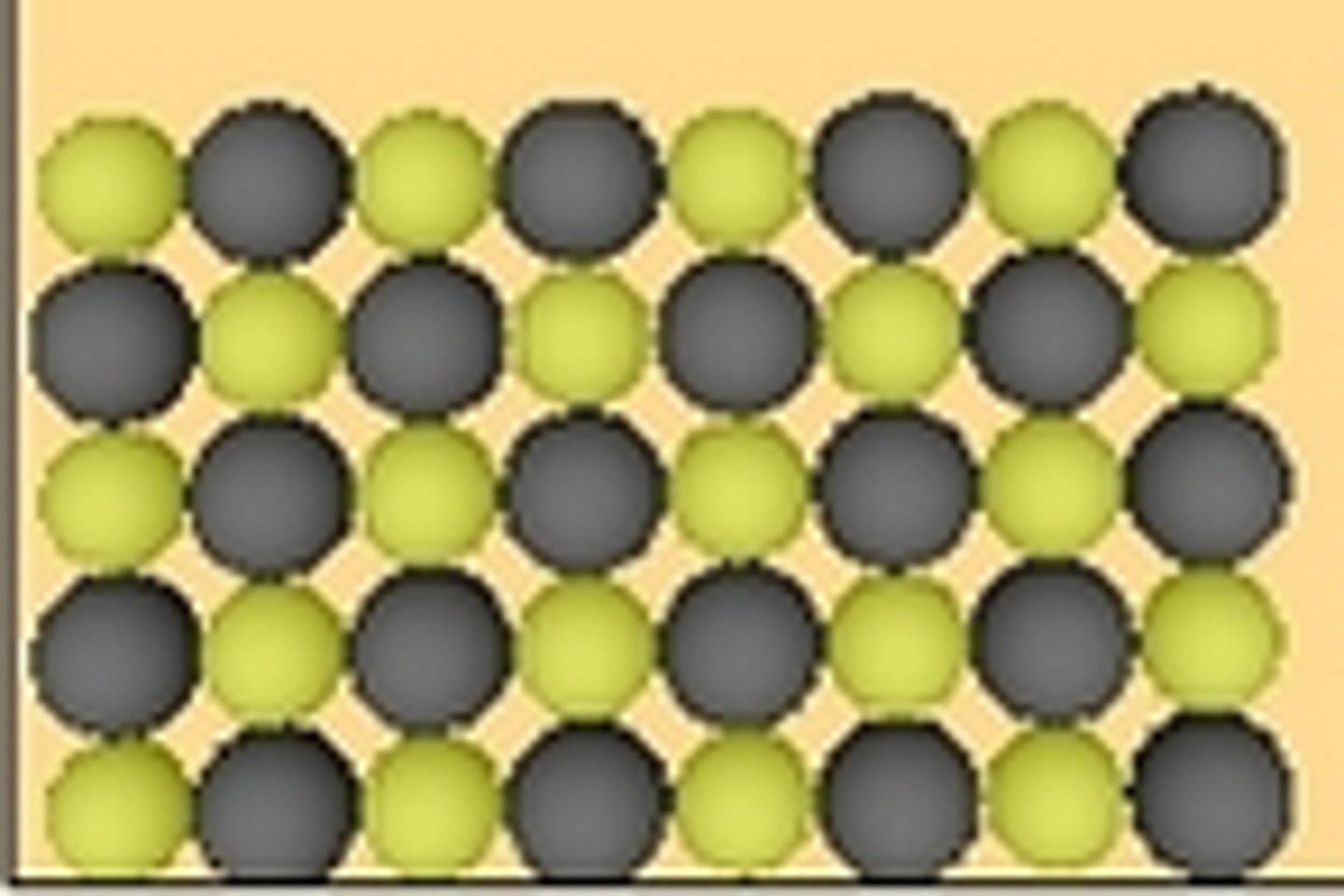
two different colors indicate solid compound
Which representation best illustrates a solid compound?
B. Evaporating water from a sugar and water mixture C. Vaporization of ethanol
Which would be a physical change?
B. 35.45 g
12. A 58.44 g sample of sodium chloride contains 22.99 g of sodium. What mass of chlorine is present?
D. Thompson's Cathode Ray
13. Which experiment's key finding was the discovery of the electron?
CaBr2
14. Which compound was made from an alkaline earth metal and a halogen?
S
18. Which element would be expected to have an ion with a -2 charge?
15.5 amu
20. What would be the average atomic mass of a hypothetical element if the element has only two isotopes? Isotope 1= 15.0 2= 17.0 Abundance%= 1=75 2=25
7 atoms
1. (6 points) How many atoms are present in one molecule of dinitrogen pentaoxide (N2O5)?
3 ions
2. (6 points) How many ions would be present in one formula unit of calcium chlorate (Ca(ClO3)2)?
ionic bonding
3. (6 points) What type of bonding exists in SnS2?
Ionic compounds are strong electrolytes when dissolved in water since the ionic bonds can break apart to make individual ions in the solution.
4. (6 points) Which statement is true?
The carbon and sulfur atoms are sharing electrons.
5. (6 points) Which statement best describes the bonding holding the carbon to the oxygen in MgSO3?
nitrogen trioxide
6. (6 points) What is the proper name for the molecule NO3?
D. Manganese (IV) oxide 6
7. (6 points) What is the proper name for MnO2?
Mg3N2
8. (6 points) What is the chemical formula for magnesium nitride?
C. N2S4
9. (6 points) What is the chemical formula for dinitrogen tetrasulfide?
46.7%
0. (6 points) A sample of silicon dioxide contains 1.00 g of silicon and 1.14 g oxygen. What is the mass percent of silicon in silicon dioxide?
0.369 moles
11. (6 points) How many moles of atoms are present in 8.00 g of Neon (Ne)?
0.204 moles
12. (6 points) How many moles of atoms are present in 1.23x1023 atoms of oxygen (O)?
9 moles
13. (6 points) How many moles of ions are present in 3 moles of calcium chloride (CaCl2)?
62.31
14. (6 points) What is the molar mass of MgF2?
A. 294.17 g/mol
15. (8 points) What is the molar mass of Al2(SO3)3?
A. 74.19%
16. (8 points) What is the mass percent of sodium (Na) in sodium oxide (Na2O)?
C. C2H5
17. (6 points) What is the empirical formula for a molecule of (CH3)3CH?
C. Cr2O3
18. (8 points) What is the empirical formula for compound that is 68.42% chromium (Cr) and 31.58% oxygen (O) by mass?
C. C4H8O4
19. (6 points) What is the molecular formula for a compound with the empirical formula of CH2O and a molar mass of 120.1 g/mol?
D. Isopropyl alcohol is the solute and water is the solvent.
(6 points). Which statement is true about a mixture made from 40.0 g of liquid isopropyl alcohol (C3H8O; 60.09 g/mol) and 60.0 g of liquid water (H2O; 18.02 g/mol) knowing the two substances form a homogeneous mixture?
1.35M
(10 points) Calculate the molarity of a solution if 37.4 g of calcium chloride (CaCl2; 110.98 g/mol) is dissolved in enough water to make 250.0 mL of solution.
20.0 mL
(6 points) Calculate the volume (in mL) of a 0.500 M sugar solution needed to make 1.00 L of 0.0100 M sugar by diluting the solution with water.
1020g
. (10 points) Concentrated hydrochloric acid (HCl; 36.46 g/mol) is 11.65 M. How many grams of HCl would be present in a 2.40 L bottle of concentrated hydrochloric acid?
Mixing two solutions produces small solid particles iii. Opening a packet of hand warmers and they get warm
. (6 pts) Which illustration provides evidence for a chemical reaction?
1,5,3,4
. (10 pts) What are the coefficients needed to balance the reaction of methanol with oxygen to produce carbon dioxide and water (coefficients listed in the same order as reactants and products in the reaction equation)?
__C3H8(g) + ___O2(g) → ___CO2(g) + ___H2O(g)
4,3,2
(10 pts) What are the coefficients needed to balance the reaction of manganese with oxygen to produce manganese (III) oxide (coefficients listed in the same order as reactants and products in the reaction equation)?
___Al(s) + ___O2(g) → ___Al2O3(s)
1. So all atoms on the reactant side are also on the product side.
2. So the Law of Conservation of Mass is represented in the equation.
(6 pts) What is the purpose behind balancing a chemical reaction equation?
Precipitation reaction
(6 pts) Classify the reaction. 3Cu(NO3)2(aq) + 2K3PO4(aq) → Cu3(PO4)2(s) + 6KNO3(aq)
combination
(6 pts) Classify the reaction. Cl2(g) + Br2(g) → 2ClBr(g)
A. PbCl2, C. Cu(OH)2
11. (6 pts) Which substance(s) is insoluble based on the solubility rules?
Al2S3
(10 pts) Predict the precipitate(s) that would be produced by mixing aqueous sodium sulfide (Na2S) with aqueous aluminum chloride (AlCl3).
A. OH-(aq) + H+(aq) → H2O(l)
What is the balanced net ionic equation for the reaction? KOH(aq) + HNO3(aq) → KNO3(aq) + H2O(l)
HBr
Identify the acid in the reaction. HBr(aq) + NaOH(aq) → NaNO3(aq) + H2O(l)
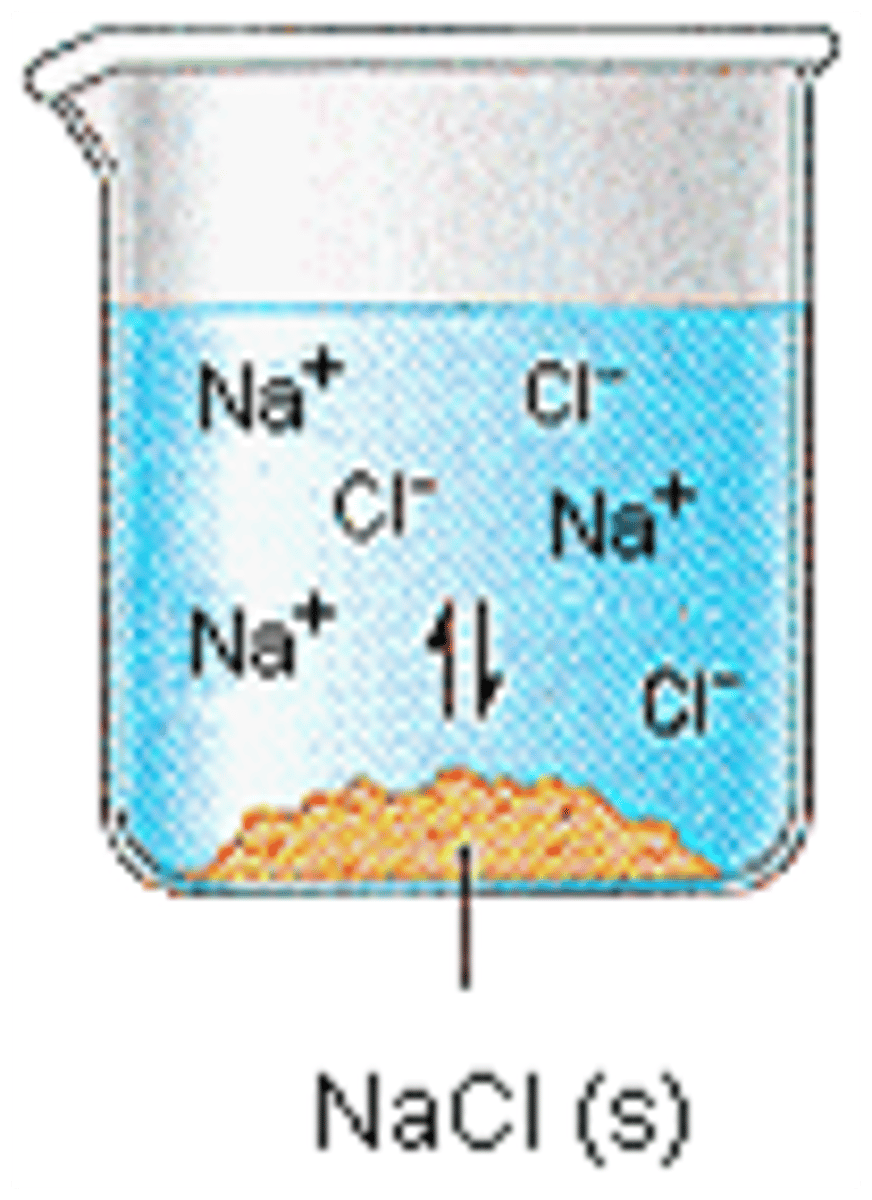
The dissolving of a weak electrolyte into water
Which statement best describes the illustration below. before all clumped together and after spread apart in pairs
4.0 moles
(8 pts) How many moles of sodium phosphate [Na3PO4] are needed to react with 6.0 moles of magnesium nitrate [Mg(NO3)2]? 3Mg(NO3)2(aq) + 2Na3PO4(aq) → Mg3(PO4)2(s) + 6NaNO3(aq)
11.9 g
10 pts) How many grams of oxygen gas (O2; 32.00 g/mol) needed to completely react with 3.33 g of butane (C4H10; 58.12 g/mol)? 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g)
O2
(6 pts) Which is the limiting reactant if 2 moles of butane (C4H10) are mixed with 10 moles of oxygen gas (O2)? 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g)
C4H10
(6 pts) Which is the limiting reactant if 100 grams of butane (C4H10; 58.12 g/mol) are mixed with 500 grams of oxygen gas (O2; 32.00 g/mol)? 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g)
C. A basketball moving toward the basket.
. (6 points) Which example would have kinetic energy?
310 KJ
An ounce of feta cheese has 75 Cal. How much energy is this in kJ?
0.391 J
Calculate the kinetic energy (in J) in a nickel (5.00 g) that is moving at 12.5 m/s.
3630 J
I) If 1.00 g of ethanol requires 2.42 J to raise the temperature by 1.00 oC, then how much energy (in J) is required to raise 50.0 g of ethanol by 30.0 oC?
-75.1
A 35.0 g block of copper (specific heat capacity of 0.385 J/g*oC) at 98.6 oC releases 2.34 kJ of heat. What would be the final temperature of the block?
0.445
A 36.4 g block of metal at 97.5 oC is placed in 125 g of water at 21.0 oC. The final temperature of the block and water is 23.3 oC. What is the specific heat capacity of the metal (in J/goC)? (Specific heat capacity of water is 4.184 J/goC)?
highest frequency= most bumps or peaks B and C
A 36.4 g block of metal at 97.5 oC is placed in 125 g of water at 21.0 oC. The final temperature of the block and water is 23.3 oC. What is the specific heat capacity of the metal (in J/goC)? (Specific heat capacity of water is 4.184 J/goC)?
0.122
Microwave ovens use electromagnetic radiation with frequency of 2.45 GHz. What is the wavelength of this electromagnetic radiation? (speed of light is 3.00x108 m/s)
C. An electron will leave the atom with 2.1x10-19 J of kinetic energy.
An atom that requires 7.1x10-19 J of energy to ionize absorbs a photon with 9.2x10-19 J of energy. Which statement describes the result?
Atoms contain energy levels with very specific energies, and electrons moving from higher to lower energy levels emit very specific wavelengths of light.
Which statement best describe how the Bohr model of the atom explains emission line spectra (the emission of very specific wavelengths of light by an atom)?
4
15. (6 points) How many electrons are in the highest energy level in a germanium (Ge) atom?
8
16. (6 points) How many electrons are in the highest energy level in a sulfide ion (S2-)?