AICE Enviro Unit 7
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Acid Deposition
is a mix of air pollutants that deposit from the atmosphere as either acidic wet deposition (pH<5.6) or acidic dry deposition
Wet Deposition
occurs when acids are dissolved in precipitation at great distances from the sources of the pollution
ex. snow, hail, or fog
Dry deposition
typically occurs close to the source of emission and causes damage to buildings and structures
ex. dust (fine particles) and gases
Acid Rain
caused by human activities
sulfur dioxide & nitrogen dioxide are released after burning fossil fuels
Wind spreads acidic solutions
Acid rain enters water systems through runoff that sinks into the ground
Plants/trees affected by acid rain
harms forests, damaging tree leaves
robs soils of essential nutrients
harder for trees to take up water
ways humans can reduce acid rain
designing cleaner power plant, using fewer fossil fuels.
reduce number of pollutants that create acid rain
Primary pollutants
are those that are directly emitted by a factory (coal powered) or automobile
ex. sulfur dioxide (SO2) Nitrogen monoxide (NO) and Nitrogen dioxide (NO2)
Secondary pollutants
are the result of primary pollutants reacting with other substances in the atmosphere
ex. Sulfuric Acids (H2SO4) and Nitric Acids (HNO3)
Sulfuric Acid Deposition
Fossil Fuels contain Sulfur compounds
Combustion of fossil fuels release sulfur dioxide gas
Sulfur dioxide gas reacts with water and oxygen in the atmosphere to form sulfuric acid
Nitric Acid Deposition
Nitrogen from the atmosphere reacts with oxygen in the high temperatures of vehicle engines to form nitrogen monoxide gas
Nitrogen monoxide gas is released into the atmosphere in vehicle emissions
nitrogen monoxide gas reacts with oxygen and water in the atmosphere to form nitric acid
Impacts of Acid deposition on Aquatic Ecosystems
Ecological effects of acid rain are most clearly seen in aquatic environments, such as streams, lakes, and marshes where it can be harmful to fish and other wildlife.
As it flows through the soil, acidic rainwater can leach aluminum from soil clay particles and then flow into streams and lakes.
The more acid that is introduced to the ecosystem, the more
aluminum is released.
Some types of plants and animals are better able to tolerate acidic waters and moderate amounts of aluminum.
Others, however, are acid-sensitive and will be lost as the
pH declines.Generally, the young of most species are more sensitive to environmental conditions than adults.
At pH 5, most fish eggs cannot hatch.
At lower pH levels, some adult fish die.
Some acidic lakes have no fish.
Even if a species of fish or animal can tolerate moderately acidic water, the animals or plants it eats might not.
Ex: Frogs have a critical pH around 4, but the mayflies they eat are more sensitive and may not survive below pH 5.5.
Impacts of Acid deposition on Vegetation and crops
High concentrations of dissolved aluminum released from soils often
enter streams and lakes.
In conjunction with rising acidity in aquatic environments, aluminum
can damage/destroy fish gills thus impairing fish respiration.
REMEMBER: Acidity = Higher hydrogen ion (H+)
concentration and lower pH.
Essential nutrients in the soil such as calcium, potassium, and magnesium can be leached from soil as it becomes more acidic. This is detrimental to plant health (both trees and crops). Results in LOWER crop yield!
Root hairs are damaged which results in less uptake of water from the soil (both trees and crops).
Both direct and indirect effects of acid deposition lead to defoliation (loss of leaves/needles).
Acid deposition can strip nutrients from trees’ foliage, leaving them with brown or dead needles/leaves.
When this happens, trees are less able to absorb sunlight to perform photosynthesis. This makes trees weak and less able to withstand freezing temperatures and disease.
Impacts of Acid Deposition on Stone and Brick Buildings
The weathering of rocks by chemicals (such as acids) is called
chemical weathering.
Acid rain makes chemical weathering happen more quickly.
As a result, buildings made from rocks/concrete are damaged. The
type of rock most quickly affected is limestone.Limestone buildings and statues (including many with great
archaeological and historical value) react with acid and simply
dissolve.
Photochemical Smog
a mixture of air pollutants and particulates, including ground level ozone, that is formed when oxides of nitrogen and volatile organic compounds (VOCs) react in the presence of sunlight
forms when sunlight drives chemical reactions between primary pollutants and atmospheric compounds, producing a mix of more than 100 different chemicals, tropospheric ozone often being the most abundant.
Because photochemical smog also includes NO2, it
generally appears as a brownish haze.
Industrial smog
is created primarily by the burning of coal (power plants)
Soot (particulate matter) and Sulfur Dioxide are produced as a result of combustion of coal
This mixture forms smog where reactions of sulfur dioxide, atmospheric oxygen, and water vapor result in sulfuric acid mixed with the smog.
Vehicle contribution
Most smog pollution in urban areas result largely from automobile exhaust
Vehicles contribute Ox, SOx, and VOCs to the atmosphere. Some of these pollutants react with sunlight, so photochemical smog pollution tends to be worse in cities with sunny climates. Ex: Los Angeles and Mexico City.
Helps promote photochemical smog
Hot, sunny, windless days in urban areas provide perfect conditions for photochemical smog to form.
On a typical workday, exhaust from morning traffic releases NO and VOCs into a city’s air.
Sunlight promotes the production of ground-level (tropospheric) ozone and other secondary pollutants, leading to a midafternoon peak in pollution.
Impacts of Photochemical smog
Photochemical smog irritates people’s eyes, noses, and throats, and over time can lead to asthma, lung damage, heart problems decreased resistance to infection, and even cancer.
Photochemical smog can lead to decreased crop yield due to decreased photosynthesis and poor plant health.
The chemical make-up also deteriorates plastics and rubber.
Strategies to manage air pollution
Reduction of Fossil Fuel Use
Reduction of Emissions:
SOx, NOx, VOCs, and Particulate Matter
Restriction of Vehicle Use in Urban Areas
Legislation
Reduction of Fossil Fuel use
Reduce demand of private car use
Mass Transit or Ride Sharing
Drive less and walk/bike/bus more
Reduce consumption of electricity through building design & renewable energy sources
small scale green power on city buildings through solar, wind, locale power stations away from urban areas
Renewable, non-pollution emitting energy sources (solar, wind, hydro) Alternative (nuclear) energy during transition to renewable energy dependency
Eat local, eat more plants, less meat - less processing
Reduction of Sulfur Dioxide
Flue Gas Desulfurization
Dry scrubbers
wet scrubbers
a flue is a smokestack
Hydrodesulfurization or hydrotreating
A catalytic chemical process widely used to remove sulfur compounds from refined petroleum products such as gasoline or petrol, jet fuel, diesel fuel, and fuel oils
Why reduce Sulfur Dioxide
One purpose for removing the sulfur is to reduce the sulfur dioxide emissions resulting from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
Reduction of Nitrogen Oxide emissions
Catalytic Converters have been around since 1975.
This device converts harmful pollutants such as carbon
monoxide and oxides of nitrogen from a combustion engine’s
exhaust into less harmful molecules like CO2, water, and nitrogen.
Sources of Volatile Organic Compound
Household products
paints, paint strippers and other solvents
wood preservatives
aerosol sprays
cleansers and disinfectants
moth repellents and air fresheners
stored fuels and automotive products
hobby supplies
dry-cleaned clothing
pesticide
Reducing Volatile Organic Compounds
increase ventilation when using products that emit VOCs
Meet or exceed any label precautions (including proper disposal)
Do not store opened contained of unused paints and similar materials within the school
Formaldehyde, one of the best-known VOCs, is one of the few indoor air pollutants that can be readily measured.
Reduction of Particulate Matter
Electrostatic Precipitators
Particles are electrostatically charged in the gas stream.
Charged particles stick to collection plates.
Clean air exits through a stack to the atmosphere.
Plates are shaken by mechanical rappers to remove particles.
Particles fall into a hopper and are moved for disposal or recycling.
Restriction of vehicle use in Urban areas
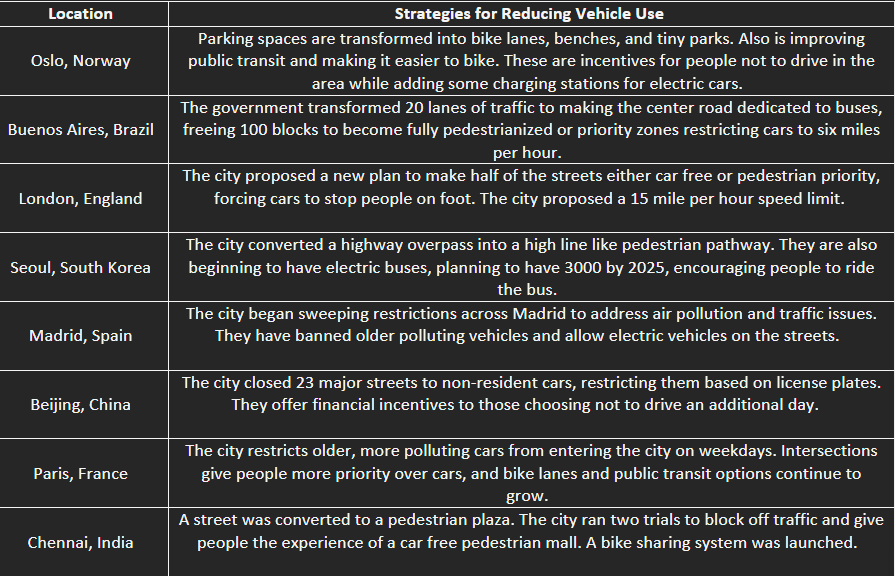
Legislation
local and national government can encourage individuals through educational campaigns and economic incentives
energy efficiency in homes
reduction of carbon footprints
reduce, reuse, recycle
replacement of chemical fertilizers with organic fertilizers
Geneva Convention on Long Range Transboundary Pollution 1979
controlling and reducing air pollution across borders by internation corporation
Montreal Protocol 1987
ban and controlled use of CFCs to slow down ozone depletion.
has been the most successful international agreement in reducing/eliminating the production and use of ozone depleting substances.
Once the protocol was signed the regulation went into effect, causing surface levels of ozone depleting substances to go down
The Ozone hole is becoming smaller because of decline in Chlorine released from Chlorofluorocarbons
Substances get high up in the stratosphere and the chlorine gets released from the chlorofluorocarbons, which is what destroys the ozone.
Hydrogen chloride in the atmosphere measured over time shows that the HCl, reactive chlorine that destroys the ozone, the levels are going down inside the Antarctic ozone hole.
Ozone levels can vary depending on temperature
If warm not as much ozone depletion, if cold more ozone depletion
Microwave Limb Sounder (MLS) shows data that ozone depletion has declined
Rio Earth Summit 1992
Agreement Agenda 21 passed with aim to cut environmental pollution and conserve and wildlife habitats
Kyoto Protocol
signed by over 100 countries to cut carbon dioxide emissions by 5%, compared with 1990 levels. Each HIC was allotted a target on emission reductions. Some LICs, such as China, were given no targets and were allowed to increase emissions
Gothenburg Protocol
Aimed to reduce pollutants and levels of acid rain and tropospheric ozone
Copenhagen Conference 2009
HICs and some LICs agreed to limit on greenhouse gas emissions. To assist LICs with the reduction, $30 billion was offered as aid by HICs, increasing to $100 billion by 2020. However, was not legally binding
Paris Climate Conference 2015
195 countries agreed to limit rise in global temperature to 2C. European Union will cut carbon dioxide emissions by 40% in 2030, the US by 28% by 2025, and China agreed their emissions will peak in 2030. Countries will meet every 5 years to discuss progress.
Polluter Pays Principle
The 'polluter pays' principle is the commonly accepted practice that those who produce pollution should bear the costs of managing it in order to prevent damage to human health or the environment.
Air Quality in the U.S.
leads to health problems, particle pollution can infiltrate blood and cause heart attacks and strokes.
Impacts economy, roughly costing three percent of its GDP in damages or $617 billion
It can cause people to cough, wheeze, and have shortness of breath.
Ozone Depletion
Step 1: Chlorofluorocarbons (CFCs) from aerosols and refrigerants are unreactive compounds and are not broken down in the troposphere.
Step 2: CFCs move into the stratosphere and break down in the presence of ultraviolet light to release a chlorine atom.
Step 3: Rapid reactions between chlorine atoms and ozone breaks down ozone (O3) into oxygen (O2), causing ozone depletion.
Step 4: Chlorine atoms remain in the stratosphere and can continue to destroy ozone.
Measuring Ozone
Ozone concentration is measured using the Dobson Unit.
An ozone hole is an area where the average concentration of ozone is below 100 Dobson Units.
The sun emits large amounts electromagnetic radiation in all directions
Visible light is the small fraction of this radiation the human eye can see
Below visible light is ultraviolet which is divided into UVA UVB and UVC
Ozone layer protects the Earth from these bands
UVA is not blocked by the ozone layer, accounting for 97% of the solar Uv energy reaching near the surface
UVB is absorbed by the ozone layer, accounting for remaining 5%
UVC is the most damaging UV radiation, is completely absorbed by the ozone in the upper atmosphere, no measurable amount reaches the Earth’s surface.
Antarctica Ozone Depletion
Factors include: temperature, polar vortex, and polar stratospheric clouds (PSCs).
Polar Stratospheric clouds, man made CFCs, and intensive heat from the sun decimates huge portions of the ozone layer.
The ozone layer is important because it blocks harmful UV radiation from the sun.
CFCs are exposed to ultraviolet radiation in the stratosphere, due to chlorine in CFCS
Montreal Protocol made to decrease CFCs
Many research stations are in the arctic to measure the cleanest air on earth and monitor the ozone layer through high resolution data
In dark winter patterns stabilize around Antarctica preventing warm air from mixing in, creating the Antarctic Polar Vortex
Impacts of increased UV Radiation
Deterioration of the ozone layer results in an increase in harmful UV Radiation reaching the Earth’s surface
cataracts and skin cancer
decreased crop yield
decreased biodiversity of terrestrial (forests) and aquatic (plankton) ecosystems
Degradation of materials used in clothing and construction
Impacts of Alternatives to Ozone Depleting substances
Alternatives to CFCs:
Hydrofluorocarbons HFCs are greenhouse gases (GHGs) commonly used by federal agencies in a wide variety of applications, including refrigeration, air- conditioning (AC), building insulation, fire extinguishing systems, and aerosols. HFCs have high global warming potential (GWP), raising concern about their impacts as they become increasingly used as replacements for ozone-depleting substances (ODS), and as economic growth spurs demand for new equipment, especially in the refrigeration/AC sector.
Fluorinated Gases (F-GHGs) include the most potent and longest lasting greenhouse gases emitted by human activities. These are emitted as a byproduct of aluminum production and electronics manufacturing.
Rowland and Molina
Initially the main hypothesis was not accepted b/c some of the auxiliary hypotheses were not supported by experimental evidence.
The hypothesis led to further research and data collection by other scientists, which confirmed that CFCs are ozone depleting.
Ultra-Low Emission
Ultra-Low Emission Zones encourage use of electric vehicles; fines for non-compliance; reduction in vehicle emissions; improves air quality / air is cleaner; especially in areas of high population;