3. passive transport and generation of membrane potentials
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
spontaneous processes in nature are driven by (blank- 3 words)
potential energy gradients
process stops when potential energy decreased ΔE
ΔE = 0 means
equilibrium
driving force of diffusion?
concentration (another form of energy) or chemical potential gradient
high to low
membranes should be permeable to solute or solution?
both! different membrane permeabilities and desired end results
T or F: rate of diffusion decreases logarithmically
true! ΔEₓ = log (CₓA - CₓB) approach 1 bc log1=0
counter ions
ionic compounds dissolve in water dissociate into their negative and positive ions; still retain strong attractive forces to one another
ex: if K+ is permeable and crosses membrane (cytosol → extracellular fluid) due to their concentration gradient, they are still close to M- bc of their charges (electrical forces are strong)
law of electroneutrality
ionic solutions must have equal numbers of (+) and (-) charges
what can make membrane polar and what does it gain?
a membrane with increasingly positive charges on the outside and negative on the inside makes the membrane polar (K= and M- example) and it gains electrical potential
one each side the similar charges repel each other so additional ion accumulation is hard
eventually K+ is stopped from going to the outside
what are the two gradients that can occur across a membrane with Cl- and K+?
concentration gradient
voltage gradient aka membrane potential (makes K+ wanna go back in)
*eventually K+ cannot follow concentration gradient due to repelling forces extracellularly; reach equilibrium
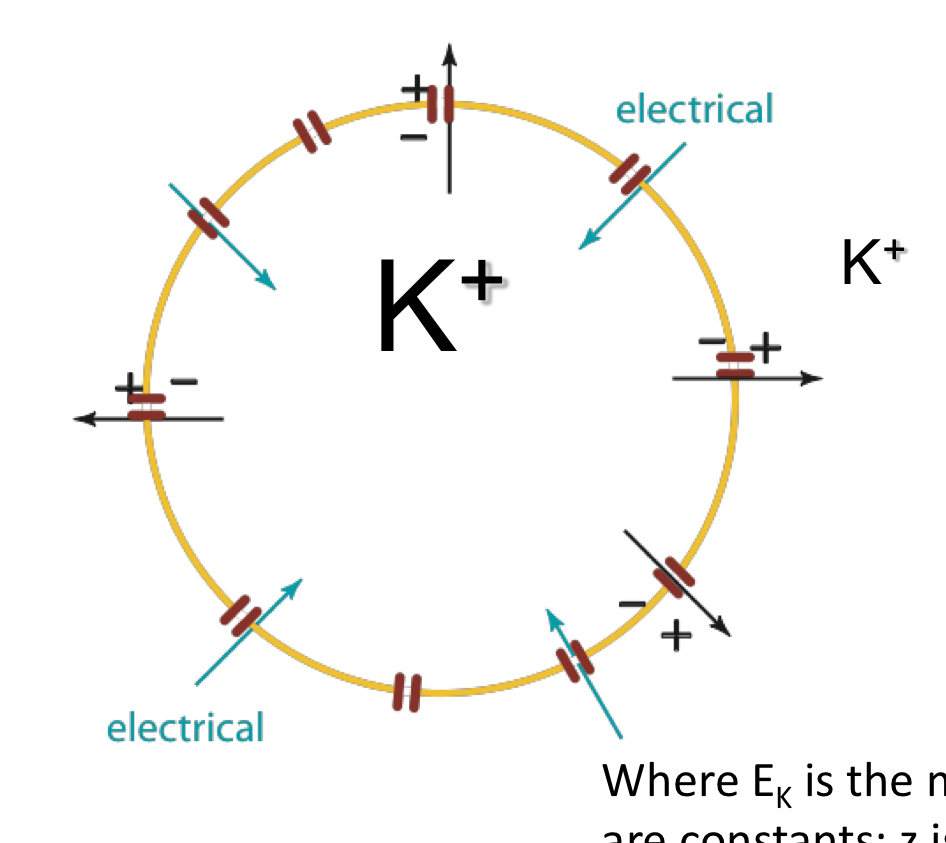
Nerst equation in relevant terms
equilibrium aka membrane potential is achieved when concentration gradient (K+ move outside cell) = electrical gradient (K+ move into cell)
T or F: the sign of E is indicative of the charge inside the cell membrane
true
E = (-) vs E = (+) means:
(-): inside cell overall negative
(+): inside cell positive
explain membrane potential
electrical charge on the membrane, measured in millivolts, “E”, has polarity and magnitude, measure with voltmeter with electrode in and out of cell
E indicative of membrane interior
nerst equation in cells
E = 60 log (C ion out/ C ion in)
for a membrane whose EK= -80mV…
… this means membrane is negative on the inside and for a cell with K+ as the only permeable ion, K+ would have a higher intracellular concentration than extracellular (K is on the inside diffusing out)
when does Nerst equation work?
only one permeable ion; to determine membrane changes at equilibrium if concentrations of permeable ion in and out of cell are known
the four important ions in neurophysiology?
K+ EK= -93mV
Na+ ENa= +65 mV
Ca2+ ECa= +120-150 mV
Cl- ECl= -89mV
more permeable ion will
leave some ions on the membrane (accumulation)
more than one ion is permeable is indicated by what E?
Em value would be between the permeability of each ion (there is a max and min)
could be closer to one of the individual potentials; the one that is closer to Em has a smaller driving force than the individual ion E that is farther away from Em
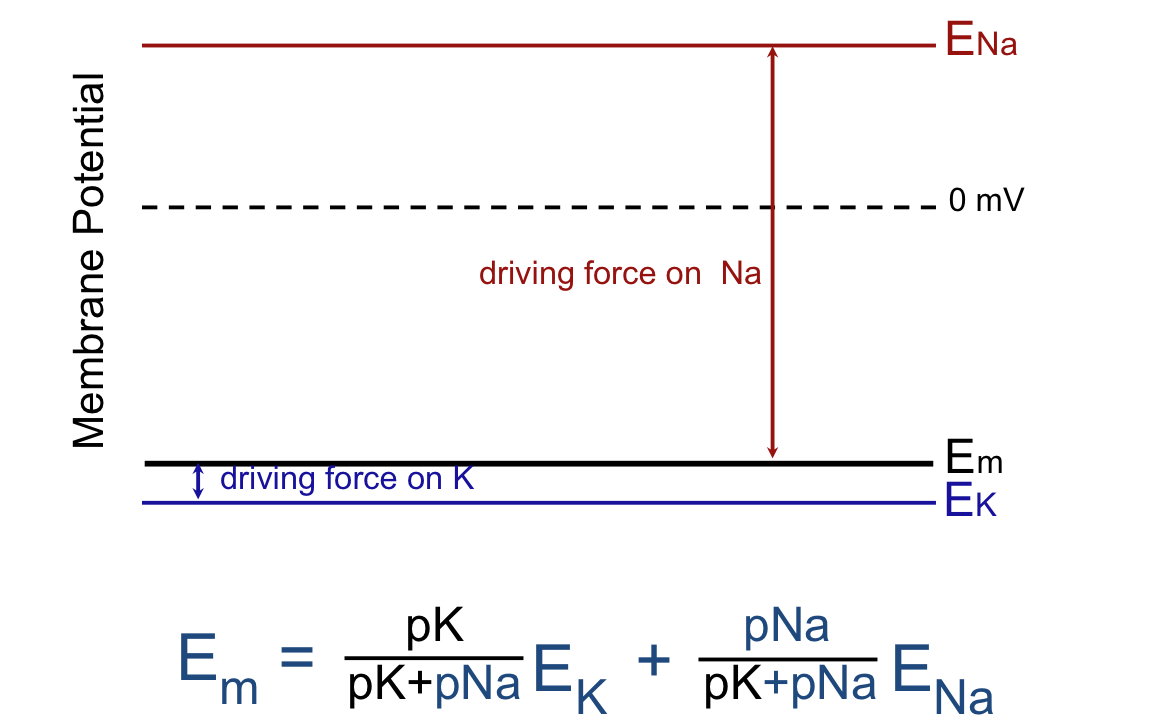
T or F: if the permeability of any ion increases, the Em will move towards the equilibrium potential of that ion
true!
what is the typical Em for a resting neuron?
-80 mV and it is called resting potential
what is happening in a resting neuron?
permeable to Na and K
Em = -90 to -70 mV meaning it has higher permeability for K ions (more K leaving and positive charge on outside and negative ions inside)
some Na and K are leaking in so diffusing down concentration gradient
Na/K pumps:
active transport
uses cellular energy to move ions against their concentration gradients
returns diffusing ions to the original sides maintaining the concentration gradients of the two ions (make K that escaped out back in and Na that escaped in back out)
NOT AT EQUILIBRIUM; ongoing steady state system
Na/K pumps move K (blank) and Na (blank)
2 K in and 3 Na out
membrane potential Em is influenced only by
permeable ions
ions move (passively or actively) down electrochemical gradients
passively
the influence of any ion on Em is proportional to that ion’s …
… relative permeability
T or F: a large proportion of all available ions move to set up/change Em
FALSE very small proportion
T or F: ionic concentration gradients do not change significantly with Em changes
true
if ENa = -70 mV
what ion is permeable
concentrated more inside cell or outside?
which direction is the ion diffusing?
what ion is permeable: K
concentrated more inside cell or outside? inside
which direction is the ion diffusing? outside