Lab H- Aldol Condensation
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is an aldol reaction?
-When two molecules of an aldehyde or ketone react with each other in the presence of a base to form a Beta-hydroxyl carbonyl compound
-formation of a new carbon-carbon bond at the alpha carbon
Where is the alpha carbon?
The carbon adjacent to the carbonyl group
Where does Aldol get its name from?
Many Aldol products contain an aldehyde and an alcohol
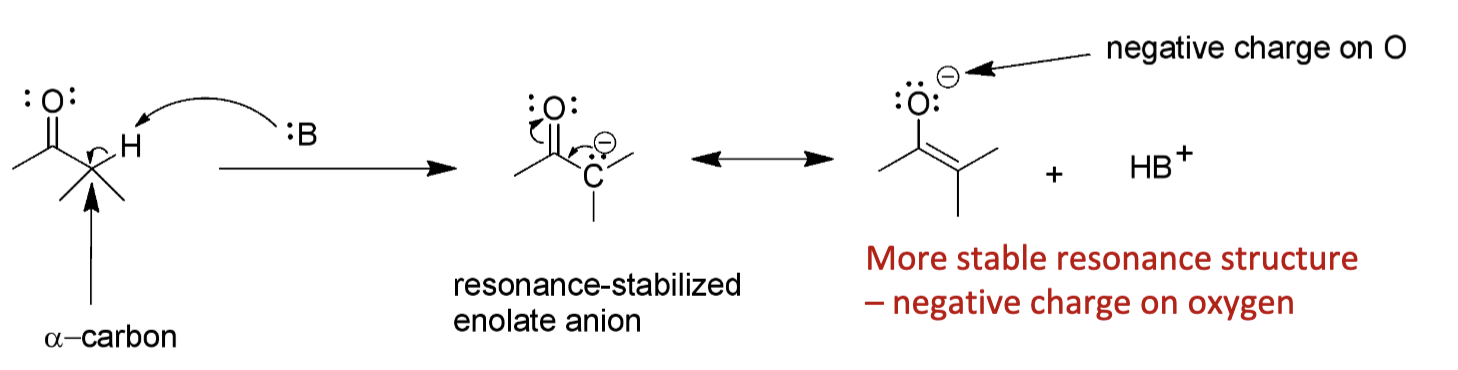
How are enolates formed?
Enolates are formed when a strong base removes a proton on the alpha-carbon
Why is the C-H bond on the alpha-carbon more acidic?
Because the resulting enolate is resonance stabilized
What is the pKa of an aldehyde/ketone? Is it more or less acidic than C-H bonds alkanes? Is it more or less acidic than O-H bonds in alcohols and acids?
Approximately 20 and its more acidic than C-H bonds but less acidic than O-H bonds and acids
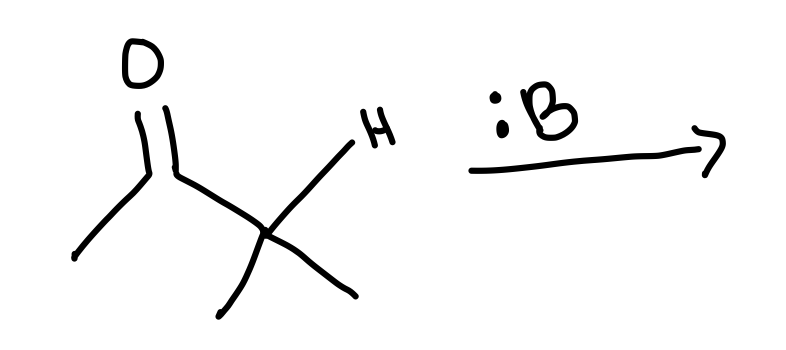
Draw the general reaction of the formation of an enolate, starting with this
What are the two types of Aldol Reactions?
Symmetrical Aldol Reactions and Crossed/Mixed Aldol Reactions
What is a Symmetric Aldol Reaction?
An Aldol reaction that occurs between two identical aldehydes or two identical ketones as the starting compound
What is a Crossed/Mixed Aldol Reaction?
Aldol reactions that can occur between two different carbonyl compounds
When is a crossed aldol reaction not synthetically useful?
When two different aldehydes have alpha hydrogens
How many Aldol products can be formed in a mixture?
4
When are crossed aldol reactions most successful?
When one carbonyl compound has no alpha-hydrogens and can’t form an enolate anion, leading to the formation of only one product
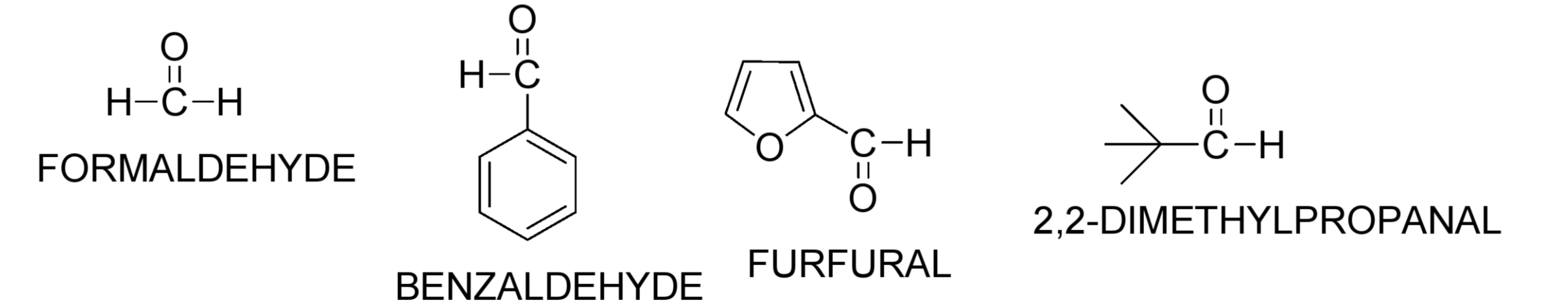
What are some examples of aldehydes with no alpha-hydrogens? Draw their structures
Formaldehyde, Benzaldehyde, Furfural, 2,2-Dimethylpropanal
What was the order the chemicals in Lab H was added?
95% EtOH
Benzaldehyde
KOH
Acetone
What type of Aldol reaction was Lab H?
Crossed Aldol Condensation
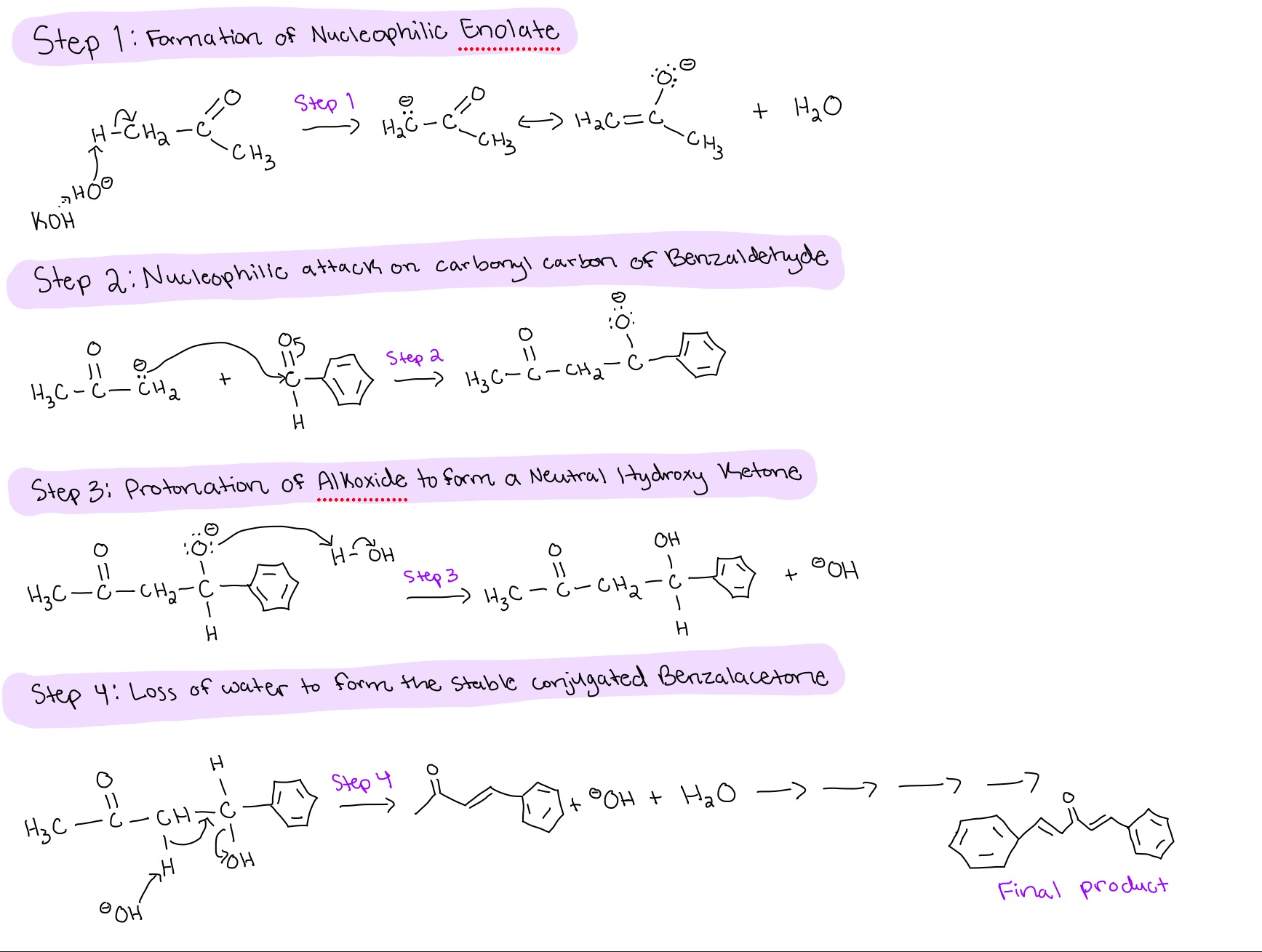
Draw the reaction of Lab H, Crossed Aldol Condensation reaction
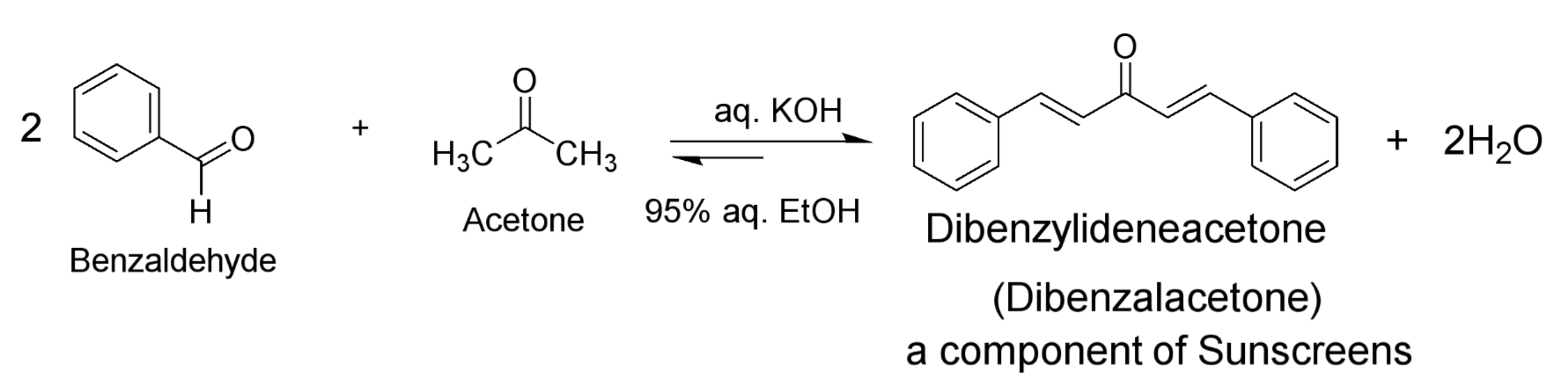
What is an application of the product formed in this Aldol reaction? (Dibenzylidenacetone) What is its significance?
Its a component of sunscreens and its significance is that conjugation allows for absorption of sunlight
Why should acetone be added last?
Because we already formed the enolate, so this prevents acetone from forming the enolate and reacting with itself
What was the limiting reactant in Lab H?
Acetone
Crossed Aldol Condensation Mechanism