Chapter 11 - Reactions of Alkyl Halides: Nucleophilic substitutions
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Types of alkyl halides
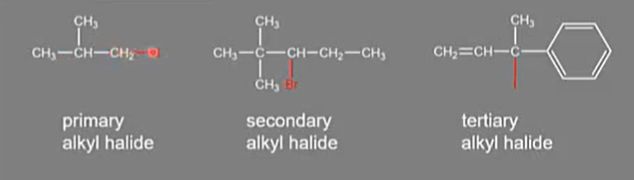
Bond polarity in alkyl halides
Big bond polarity.
Nucleophilic substitution reaction

Two types of reaction mechanism with alkyl halides
Sn2
Sn1
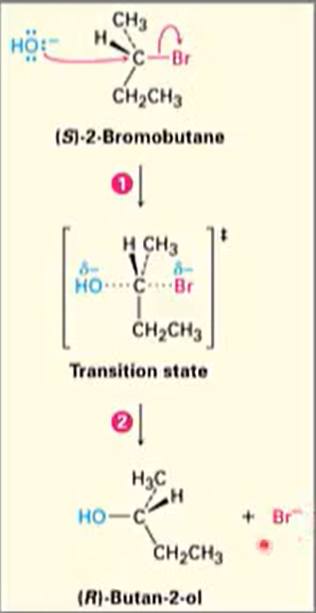
SN2 reaction
Reaction takes place in a single step
Incoming nucleophile appraoches from a direction 180o away from the leaving halide ion, thereby inverting hte stereochemistry at carbon.
e.g. Reaction goes from S to R
Reaction rate formula for SN2 reaction
Second order reaction
Reaction rate = Rate of disappearance of reactant
= k x [RX] x [OH]
(Both concentrations are important hence Sn2 reaction)
What kind of reactions are Sn2
Substitution nucleophilic and bimolecular.
Characteristics of the SN2 reactions (substrate)
The substrate: substrate needs to be available, therefore a bulky substrate prevents the easy approach of the nucleophile, making bond formation difficult.
Tertiary is less reactive in SN2 reaction, primary is most reactive.
This is due to steric effects.
Characteristics of the SN2 reactions (Nucleophile)
An Sn2 reaction requires a strong nucleophile
Nucleophilicity roughly parallels basicity when comparing nucleophiles that have the same reacting atom
e.g. OH- is more nucleophilic than CH3CO2-
Nucleophilicity usually increases going down a column of the periodic table (opposite of electronegativity because it wants to give e- instead of take)
HS- is more nucleophilic than HO-
Halide reactivity order from most to least reactive is I- > Br- > Cl-
Negatively charged nucleophiles are usually more reactive than neutral ones
Characteristics of the SN2 reactions (leaving group)
Best leaving groups are those that best stabilize the negative charge in the transition state
Weak bases such as Cl- and tosylate ion make good leaving groups, while strong bases such as OH- and NH2- make poor leaving groups.
Characteristics of the SN2 reactions (Solvent)
You should use more polar solvents for a quicker and more reactive reaction
Aprotic solutions are better for Sn2
Protic: contains OH or NH group. A protic does not.
Characteristics that affect Sn2 reaction
Solvent
Leaving group
Nucleophile
Substrate
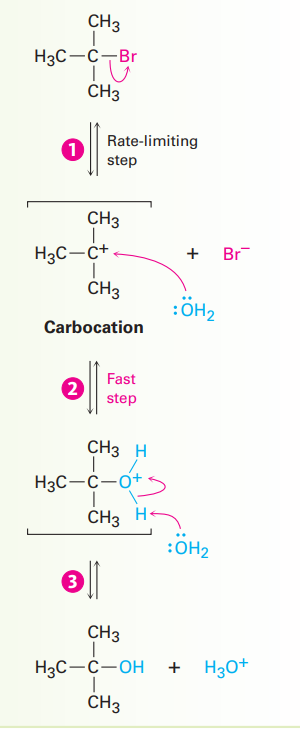
SN1 reaction
Involves more than one step
Step 1: Carbon - halogen bond is broken. Which forms a carbon cation.
This step is known as the rate-limiting step because it is the slowest step of a multi-step chemical reaction
Step 2: A nucleophile that forms a new bond.
Equation rate of reaction of Sn1
first order reaction
the concentration of the nucleophile does not appear in the rate equation
Reaction rate = rate of disappearance of alkyl halide
= k x [Substrate]
Sn1 = unimolecular nucleophilic substitution
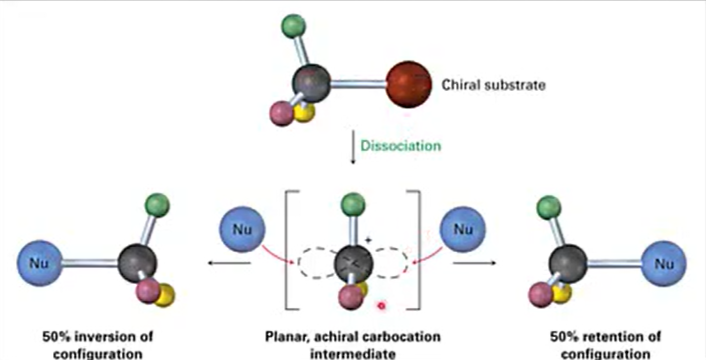
Stereochemistry in SN1 reaction
Gives racemic mixtures
50/50% ratio
Enantiomers
Characteristics of Sn1 reaction (substrate)
The more stable the carbocation intermediate the faster the reaction
Which is more stable when more substituted (tertiary)
Characteristics of Sn1 reaction (The leaving group and nucleophile)
Leaving group: same as in Sn2.
Best leaving groups are those that best stabilize the negative charge in the transition state
Weak bases such as Cl- and tosylate ion make good leaving groups, while strong bases such as OH- and NH2- make poor leaving groups.
The nucleophile: does not affect the SN1 reaction rate
Characteristics of Sn1 reaction (Solvent)
More polar = more reactive
Because polar solvents stabilize the intermediate carbocation
Characteristics of Sn1 & Sn2 reactions (specific substrates)
Allylic and benzylic substrates are particularly reactive in Sn2 and Sn1 reactions
Allylic and benzylic C-X bons are a bit weaker than the corresponding saturated bonds.
Predicting if a Sn2 or Sn1 reaction will take place
Sn1 | Sn2 | |
Substitution | More substitution because there is an intermediate with a carbocation that is more stable when more substituted. | Less substitution, primary halides are better. If it becomes more substituted the reaction will proceed slower. |
Allyl and benzyl | Allyl and benzyl substituted substrates are very reactive in Sn1 because they have resonance and are more stable in intermediate | Allyl and benzyl can also react in Sn2 because they are primary halides. |
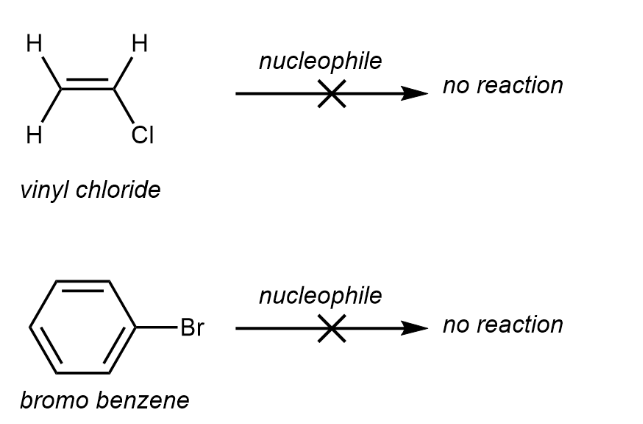
Vinyl and aryl substrates | Do not participate in reaction - due to double bond it is hard to form carbocation | Do not participate in reaction - It is hard for nucleophiles to approach the electron-rich pi bond. |
Solvent | Is very dependent on solvent, usually has protic solvent (has OH or NH) More polar = better | Not as dependent on solvent, usually has aprotic solvent Less polar = better |
Vinyl and aryl substrates
When halides are directly attached to a sp2 hybridized carbon.
What does it mean if a molecule is polarized?
it means that there is an uneven distribution of electron density across the molecule, leading to a partial separation of electric charges.
Effect of nucleophile on rate of reaction for Sn1 and Sn2
Sn1 has no effect because it is not involved in the rate determining step.
Sn2 has effect because it is involved in rate determining step.
How can an elimination reaction occur?
When a nucleophile/Lewis base react with an alkyl halide
Characteristics of elimination reactions
Elimination reaction almost always give mixtures of alkene products (even though one is more stable and will be produced more (zaitzevs rule)
E2 reaction
Occurs when an alkyl halide is treated with a strong base
Reaction takes place in a single step through a transition state in which the double bond begins to form at the same time the H and X groups are leaving
Second order reaction, both base and alkyl halide take part in the rate limiting step
Rate= k x [RX] x [Base]
E1 reaction
Two steps
First step rate-limiting
Carbocation intermediate is present
The more stable the carbocation intermediate the faster the E1 reaction
There is no geometric requirement on the E1 reaction (no periplanar geometry necessary)
The halide and the hydrogen are lost in separate steps
E and Z alkene will be formed (double bond is formed)
E1cB reaction
Unimolecular elimination reaction in which the C-H bond breaks before the C-X bond, giving a carbanion intermediate
Common in substrates that have a poor leaving group, such as -OH, two carbons removed from a carbonyl group, HO-C-CH-C=O
The carbonyl group makes the adjacent hydrogen unusually acidic by resonance stabilization of the anion intermediate