Human Pathology HLSC 4P95
1/164
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
165 Terms
What are the Greek Origins of Pathology?
the study of disease (pathos = suffering, logos = study)
Who is the father of modern pathology?
Rudolph Virchow → discovered cell theory
Cellular pathology
All diseases originate at the cellular level
What is Pathology?
The study of the structural, biochemical, and functional changes in cells, tissues and organs that underlie disease
What does Pathology attempt to explain?
the whys of the signs & symptoms manifested by patients while providing a rational basis for clinical care & therapy
What is General Pathology?
Common reaction of cells and tissue to injury (acute inflammation in response to an infection)
What is Systemic Pathology?
Examines alterations and underlying mechanisms in origin-specific diseases (ischemic heart disease)
What are the 4 aspects of disease?
Etiology 2. Pathogenesis 3.Morphological Changes 4. Clinical Manifestations
What is Etiology?
the cause of disease (genetic / acquired)
What is pathogenesis?
biochemical and molecular mechanisms of a disease’s development
Define Morphological Changes
Structural alterations induced in the cells, tissues and organs of the body
Define Clinical Manifestations
Functional consequences which led to clinical symptoms and signs
All diseases are related to what?
disturbances in cell function (in organelles)
What are the Housekeeping functions of cells
Protection from the environment
Nutrient acquisition
Communication
Movement
Renewal of senescent molecules
Molecular catabolism
Energy generation
Cellular communication: Autocrine
Secretion from the cell attaches to own surface receptors (ex: T-cell activation)
Cellular communication: Paracrine
Closely adjacent cells act on one another (ex: biogenic amines)
Cellular communication: Endocrine
hormones are secreted reach target via blood stream (ex: insulin)
Homeostasis
The maintenance of a “steady state” by means of physiological feedback control mechanisms
(Greek, meaning “standing” or “staying similar”)
What alters Homeostasis and what does that cause?
External stimuli can alter homeostasis
Imbalance in homeostasis can cause = Cell injury (reversible or irreversible)
Reversible Cell Injury: Cellular Swelling
Cell response that remains within the range of homeostasis due to an acute exposure to stimuli
What are examples of Hydropic Changes
A. Normal microvilli
B. Swollen microvilli
C. Invagination of cell membrane
D. Swollen mitochondria; dilation of rough endoplasmic reticulum
E. Loss of cell-to-cell contact
What happens if cell injury persists?
leads to irreversible cell injury
Irreversible Cell Injury results from what?
from chronic exposure to insults that the cell cannot recover from
Irreversible Cell Injury is characterized by what?
by nuclear changes or loss of cell integrity (A. Pyknosis B. Karyorrhexis C. Karyolysis )
What is Pyknosis
the condensation of chromatin
What is Karyorrhexis
the fragmentation into smaller particles "nuclear dust"
What is Karyolysis
dissolution of nuclear structure and lysis of chromatin by enzymes (DNase and RNase)
What are some causes of cell injury?
1. Hypoxia: reduced availability of O2 2. Anoxia: complete lack of O2 3. Toxins 4. Microbes 5. Mediators of inflammation 6. Immune reactions 7. Genetic and metabolic disorders
What are some causes of Hypoxia/Anoxia
1. Airway obstruction (choking)
2. Impeded O2 exchange at the lungs (pneumonia)
3. Inadequate O2 transport in blood (Low RBC count, anemia)
4. Blockade of cellular respiration and oxidative phosphorylation (cyanide poisoning)
Definition of Hypoxia/Anoxia: post perfusion injury
Over supply of oxygen once the obstruction is removed damages local cells as a result of oxygen radicals or reactive oxygen species (ROS)
What are the oxygen species that cause post perfusion injury
Hydrogen peroxide (H2 O2 )
Superoxide (O2-)
Hydroxyl radicals (OH•)
What are the types of toxic cell injuries
direct and indirect toxins
Direct toxins of toxic cell injury
heavy metals (i.e., mercury) leads to the disruption of S-S bonds
alters protein structure, can lead to inactivation of cytoplasmic enzymes
Indirect toxins of toxic cell injury
Must be metabolically activated carbon tetrachloride (component of metal polish) is not toxic itself
When consumed, metabolized to carbon trichloride (a free radical) which damages cells membranes
Microbial Pathogens: Bacteria produce toxins
Inhibit cellular functions such as protein synthesis or respiration
E.g., exotoxins causing food poisoning
Microbial Pathogens: Viruses invade cells and “kill from within”
Disrupt nuclear or plasma membrane
ultimately leads to cell death either from viral expulsion or immune system
What are cell adaptations?
They are functional & structural responses to changes in physiological states
What causes cell adaptations?
Result from prolonged exposure to adverse or exaggerated normal stimuli
Cell Adaptations: Atrophy
Decrease in size of cell, tissue, organ, or entire body
Physiological & predictable (age-induced)
Causes of Pathological Atrophy
Lack of nutrition
Chronic ischemia
Denervation
Inactivity
Cell Adaptations: Hypertrophy
Increase in size of cell, tissue, organ, or entire body
hypertrophy of the heart during hypertension
hypertrophy of skeletal muscle in body builders
hypertrophy is often combined with hyperplasia
Cell Adaptations: Hyperplasia
Increased number of cells found within a t issue or organ
Endometrial hyperplasia caused by estrogen
Benign prostatic hyperplasia in elderly men
Callus on hands or on heels
Cell Adaptation: Metaplasia
Characterized by the change of one cell type into another
Prolonged cigarette smoke irritation results in normal ciliated columnar bronchial epithelial cells to turn into squamous epithelium
Cell death occurs through 2 mechanisms, what are they?
Necrosis
Apoptosis
Necrosis
Exogenously induced (come in contact with some form of stimuli)
Localized death of tissue in living organisms
Apoptosis
Endogenously induced
Programmed cell death of single cells within an organism
Active form of cell death mediated by intracellular programming
Energy-dependent process that activates so-called “suicide” genes and synthesis of proteolytic enzymes
Coagulative Necrosis
Most common form of necrosis (often caused by anoxia)
Characterized by rapid inactivation of cytoplasmic hydrolytic enzymes
Prevents the lysis of tissue, which retain their original form
Typically involves solid internal organs, such as the heart, liver and kidneys
Liquefactive Necrosis
Dissolution of tissues (Become soft and diffluent)
Most common in the brain, where cells lose their contours and are “liquefied” (Semifluid mush)
Typically due to brain infarcts
Consequence of leukocytes releasing lytic enzymes which turn solid tissue into liquid pus
Caseous Necrosis
Special form of coagulative necrosis with limited liquefaction
Center of a tuberculosis granuloma becomes necrotic (mycobacteria)
Tissue is yellow-white and appears as a “cheesy” consistency
Common in fungal infections
Enzymatic Fat Necrosis
Special form of liquefactive necrosis caused by the action of lipolytic enzymes
Limited to fat tissue, usually around the pancreas (Pancreatic enzymes degrade adjacent fat t issue into glycerol and free fatty acids (FFA))
FFA bind calcium forming calcium soaps (Liquified necrosis with calcium soaps)
Dystrophic Calcification
high accumulation of free fatty acids
Secondary changes
Necrotic tissue attract calcium salts in the absence of systemic mineral imbalances
What is inflammation?
a non-specific, predictable response that can be acute or chronic
Cardinal signs of inflammation
1. Calor – heat
2. Rubor – redness
3. Tumour – swelling
4. Dolar – pain
5. Functio laesa – loss of function
Pathogenesis of Inflammation Involves
1. Circulatory changes
Vascular changes
Mediators of inflammation
Induced cellular response
Circulatory Changes: 1st response to injury involves what?
Transient vasoconstriction of arteriolar smooth muscles followed by vasodilation, Active hyperemia, Slowdown of circulation
formation of rouleaux
1. Margination of WBC
2. Adhesion of platelets
3. Pavementing of WBCs
Vascular Changes
1. Increased hydrostatic pressure
2. Slowing down of the circulation
3. Adhesion of leukocytes and platelets to endothelial cells
4. Release of soluble mediators of inflammation
Mediators of Inflammation (2 classes)
• Cell-derived (stored) • Plasma-derived (require activation)
Arachidonic Acid Derivatives
1. Phospholipases break down membrane phospholipids to arachidonic acid (AA)
2. Arachidonic acid feeds into either the cyclooxygenase, lipoxygenase or cytochrome P450 pathway
3. Cell type, type of stimuli and several other factors determine how AA is metabolized
Bradykinin
Plasma protein with similar action to histamine but slower acting Activation pathway: Hageman factor > kallikrein > kininogen > bradykinin Induces pain (dolar) Sensitization of nerve endings Hageman factor also acts on clotting, fibrolytic systems of the blood
Histamine
Stored in cytoplasmic granules of platelets, basophils, eosinophils, and mast cells
4 different receptors
Complement System Outcomes
1. Opsonization (C3b): facilitated phagocytosis of bacteria 2. Anaphylaxis (C3a, C5a): act on endothelial cells and cause histamine release – increase vascular permeability 3. Chemotaxis: migration of leukocytes 4. Cell lysis (C5-C9): formation of the membrane attack complex
Cellular Responses
Transudate, Exudate, Emigration of Leukocytes
Emigration of leukocytes
A. Adhesion of PMNs to endothelial cells (margination)
B. Insertion of cytoplasmic pseudopods between junctions of endothelial cells
C. Passage through the basement membrane (diapedesis)
D. Amoeboid movement from the vessel to the site of inflammation (chemotaxis)
E. Phagocytosis (or other cellular functions)
Cells of Inflammation
Granulocytes (Neutrophils, Eosinophils, Basophils, Mast Cells)
Macrophages
Adaptive lymphocytes
Innate lymphocytes
Platelets
Classification of Inflammation
1.Duration: acute of chronic 2.Etiology: infectious, chemical, physical, foreign bodies, or immune causes 3.Location: localized or widespread (systemic) 4.Pathological features: morphology
Morphology of Inflammation
serous exudate = clear fluid
fibrinous = large plasma proteins
purulent = pus forming bacteria, forms abscess
ulcerative = defect involving the epithelium
pseudomembranous = Combination of ulcerative inflammation and fibrinopurulent exudation
granulomatous = cell-mediated hypersensitivity reaction
Purulent Inflammation
Abscess: accumulation of pus in a newly formed t issue space
Sinus: cavity at the previous site of an abscess which drains to the surface or the tissue
Fistula: channel formed between two preexisting cavities, hollow organs, preexisting cavity and the surface of the body
Caseous Necrosis
Special form of coagulative necrosis with limited liquefaction Center of a tuberculosis granuloma becomes necrotic Tissue is yellow-white and appears as a “cheesy” consistency Also common in fungal infections (histoplasmosis)
Healing and Repair
Continuously dividing cells (stem cells) • Labile cells ex., epithelial cells that make up the skin and gastrointestinal tract
Quiescent facultative mitotic cells (stable cells) • Adult stem cells, muscle cells (satellite cells)
Nondividing postmitotic cells (permanent cells) • Neurons, myocytes, some kidney cells and adipocytes
Cells Participating in Wound Healing
Polymorphonuclear (PMN) leukocytes
Macrophages
Wound Healing — First intention (sharp, sterile, surgical wounds)
A. Incision site
B. Formation of scab & scavenger action of PMN leukocytes
C. Formation of granulation tissue
D. Scarring
Wound Healing — Secondary intention (large defects, infected wounds)
• Myofibroblasts cannot close the wound and infection can be present • Granulation tissue exposed • Prolonged healing • Scaring is more prominent
Determinants of Wound Healing
1. Site of wound (tissue type)
2. Size of the wound
3. Mechanical factors (margins, movement)
4. Infection
5. Circulatory status (ischemia = poor healing)
6. Nutritional and metabolic factors
7. Age
Complications of Wound Healing
Deficient scar formation
Excessive scar formation (keloid)
What are the 3 layers of host defense
physical barrier
innate immune response
adaptive immune response
What does the physical barrier of host defense consist of
Mechanical barriers; epidermis, ciliated cells
Chemical barriers; protective proteins and chemical secretions
Properdin in plasma activates complement system
Lysozymes in body fluids (saliva, tears, respiratory tract), kill bacteria
What does the innate immune response of host defense consist of
Phagocytic cells (neutrophils, macrophages, dendritic cells, natural killer cells)
What does the adaptive immune response of host defense consist of
Lymphocytes (T and B cells)
Cells of the immune system originate from where?
Originate from hematopoietic stem cells in the bone marrow
What are the 2 lineages of stem cells from bone marrow
1. Lymphocytes: lymphoid cells (primary cells)
2. Nonlymphoid cells (myeloid): PMNs, eosinophils, basophils, macrophages, megakaryocytes
Describe Innate Immunity
• Inherited and operational at birth
• Fast to act and nonspecific → Recognizes conserved pathogen patterns (PAMPs)
• Response to repeat infection remains relatively the same → Trained immunity (epigenetic changes)
What 3 mechanisms does Innate Immunity use to protect the body?
1. Initiating inflammation 2. Combating infections (bacterial, fungal etc.) 3. General response to damaged cells
What are some examples of innate immunity cells
Neutrophils Basophils Eosinophils Mast cells Macrophages Natural killer cells Dendritic cells
Describe Adaptive Immunity
AKA acquired immunity
• Ability to distinguish “self” from “non self”
• Specific response elicited by antigens requires time (5-7 days) → “Lock and key” receptor-antigen interactions
• Immunological memory → rapid and effective response upon subsequent exposure to the same pathogen
• Mediated by T and B cells; antigen specific receptors and antibodies
Where do T cells mature?
thymus (thymus = T-cells)
Where do B-cells mature?
Bone marrow (B cells = Bone marrow)
What are the sites of lymphoid maturation?
primary lymphoid organs
Secondary lymphoid tissue =
site of lymphoid cell activation (spleen, lymph nodes, peyers patches, adenoids, tonsils)
Cluster Differentiation (CD) antigens
• T and B cells cannot be distinguished based on morphological differences
• CD antigens – unique markers on the cell surface
• Expressed during lymphocyte development and when mature
• Markers specific to T cells (CD3, CD4, CD8) and B cells (CD19)
• Characterized via fluorescence activated cell sorting
T-Lymphocytes
T helper cells, Cytotoxic T cells, Healthy CD4
Natural Killer (NK) Cells
• Similar surface markers to T cells but do not have T cell receptor gene rearrangement
• Mediate innate immune functions and are not involved in T/B cell reactions
• Similar function to T cells – react to virus infected cells and kill tumor cells/transplanted cells
• Contain lytic granules to destroy foreign substances
B-Lymphocytes
• Essential for the production of antibodies
• Activated B-cells differentiate into plasma cells
• Plasma cells have increased ribosomes and RER for protein production/release
Antibodies
• Serum proteins secreted by plasma cells
• Comprised of 4 polypeptide chains → 2 light chains and 2 heavy chains
• Fab (variable) region is antigen specific
• Fc (constant) region
5 classes of immunoglobulins
IgM, IgG, IgA, IgE, IgD
What are the functions of Antibodies
1. B cell receptors
2. Opsonin – increase phagocytosis
3. Neutralizes antigen by binding
4. Directs immune cell effector function
5. Activates mast cells during allergy
6. Activates complement
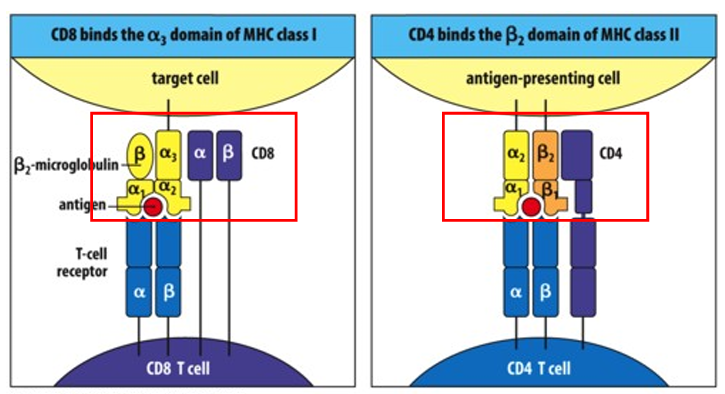
Major Histocompatibility Complexes (MHC) — What are the 2 groups?
1. MHC class I: all nucleated cells, receptors for CD8 activation – presents intracellular antigens (viruses, cancer).
2. MHC class II: present on specialized cells to bind to CD4, links macrophages to helper T cells
What are Hypersensitivity Reactions?
Abnormal immune response to exogenous antigens or endogenous auto-antigens
What are the types of Hypersensitivity Reactions
Type I: Immediate anaphylactic or atopic (allergies)
Type II: Cytotoxic antibody mediated
Type III: Immune complex mediated
Type IV: Cell mediated, delayed type
What is the 2 step process of Type I Hypersensitivity
1. Primary exposure (sensitization): primes the immune response to produce IgE antibodies that bind to mast cells
2. Secondary (subsequent exposure): Results in mast cell activation
Immediate response i.e., mast cell degranulation (histamine)
Late phase (6-9 hours) cytokines, arachidonic acid (leukotrienes, prostaglandins