SCH3U - Atomic Models and Periodic Table Practice Test Review Sept. 2023
1/64
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
In science, current available data is used to develop a model of how something works. As new data is presented, a good model evolves and changes.
Discuss the historical development of the model of the atom up to (but not including) the Bohr Model.
In 1808, Dalton ‘re-invented’ the atomic theory and stated that all matter has a smallest piece that can’t be broken down; essentially proposed atoms in a modern, scientific way. He grounded chemistry in 3 basic laws: Conservation of matter, definite proportions, multiple proportions. He came up with the Billard Ball Model (sphere)
In 1897, Thomson used a magnetic field and electric field to calculate ‘charge to mass’ ratio — the amount of charge per unit mass of the particles in the cathode ray. These couldn’t be atoms, they are subatomic particles. Thompson made the following conclusion that the cathode ray is composed of negatively charged particles, they must exist as a part of the atom as their mass is smaller than Hydrogen (the smallest atom) and they can be found within ALL elements. These particles allowed electricity to flow, thus they were called electrons and the Plum Pudding Model was born. It assumes there are no +ve particles so they are helped in a positive ‘cloud’ that is spread at equal density and the electrons are spread throughout.
In 1911, Rutherford discovered 3 types of radiation emissions (alpha, beta, gamma) which are energetic particles that can penetrate matter. He conducted a gold foil experiment, emitting alpha particles toward a thin piece of gold, expecting it to go straight through. 98% went straight, 2% went straight and then deflected at large angles, and 0.005% bounced off backwards. He concluded that the atom was mostly space since almost all went through, but there was a dense particle (we know as the nucleus) that is small in volume but large in mass. Rutherford came up with the beehive model that has very small -ve charged electrons zipping around a dense nucleus containing protons and neutrons.
Explain how Bohr’s model is different from Rutherford’s model.
Bohr’s model has the same basics as Rutherford’s model, as both of them have nuclei and electrons. Howeer, in Rutherford’s model of the atom, e-’s were zipping around everywhere, similar to bee’s in a beehive. Bohr took Einstien and Plank’s ideas of light, quantized the and organized the e-’s into orbits. This means e-’s can only exist in allowable orbits, not in between or zipping around. They also could only have specific amounts of energy in their specific orbits. Bohr’s model can be compared to planets orbitting the sun, for it is organized, unlike bees in a beehive like Rutherford’s model.
State the trends in atomic radius: across the table (L-R) then down the table. Explain why the trends follow these patterns?
Down a group, elements have the same Zeff and valance structure, but have an increasing amount of mainshells. Across a period, elements ahve their valance electrons in the same mainshell as well as the same core configuration. Zeff goes up by one and each element and the number of valancne e also go up by one.
Across the table from left to right, Zeff is increasing, so while you are adding both e- and p+, each successive atom has one more p+ pulling on the e-. The added electron's are all in the same mainshell, and the increase in Zeff pulls the electron’s of that mainshell closer and closer to the nucleus, thus the atoms get smaller
Down a group, atoms get larger: This is because each step down a group means the addition of one more mainshell, so valance electrons occupy mainshells further away from the nucleus.
What is the difference between ionization energy and electron affinity?
Ionization energy is the minimum energy needed to remove an electron from an atom (g), otherwise, how strong an atom is holding it’s valance electrons.
Electron Affinity is an energy chage in an atom when a neutral atom gains and electron (forms an anion)
(metals tend to lose e and from cations: low electron affinity, and non-metals tend to gain electrons: high electron affinity.
Why does ionizaation energy increase left to right in a period on the periodic table?
While the valance electron’s are still in the same valance shell, Zeff increases and the atomic radius decreases. Overall, these two factors mean that it takes more energy to remove an e.
The atomic radii of nitrogen, oxygen and fluorine are 70 um, 66 um and 64 um respectively. One would expact that out of the threem the atom with the highest electronegativeity would be…
Fluorine.
The unstable isotope of uranium-239 decays over time into the stable lead-206. The parent U-239 first undergoes an a-decay then a B-decay. Using proper atomic notation, write the balanced nuclear equation for these first two steps in the series.
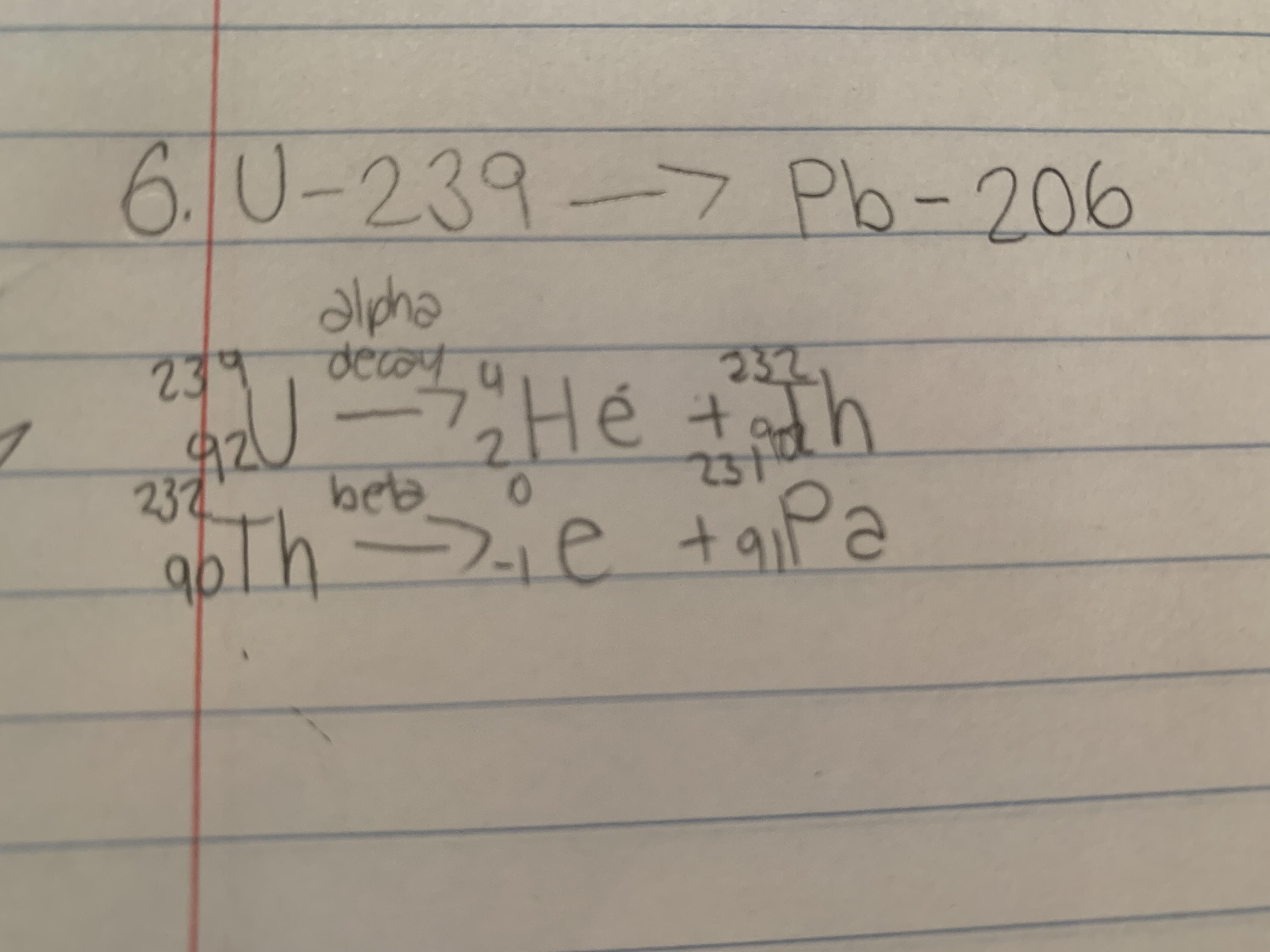
`Suppose potassium has three common isotopes, with the masses and isotopic abundances shown
P-39: 93.3%
P-40: 6.6%
P-41: 0.1%
Determine the average atomic mass of potassium that would be reported on the periodic table.
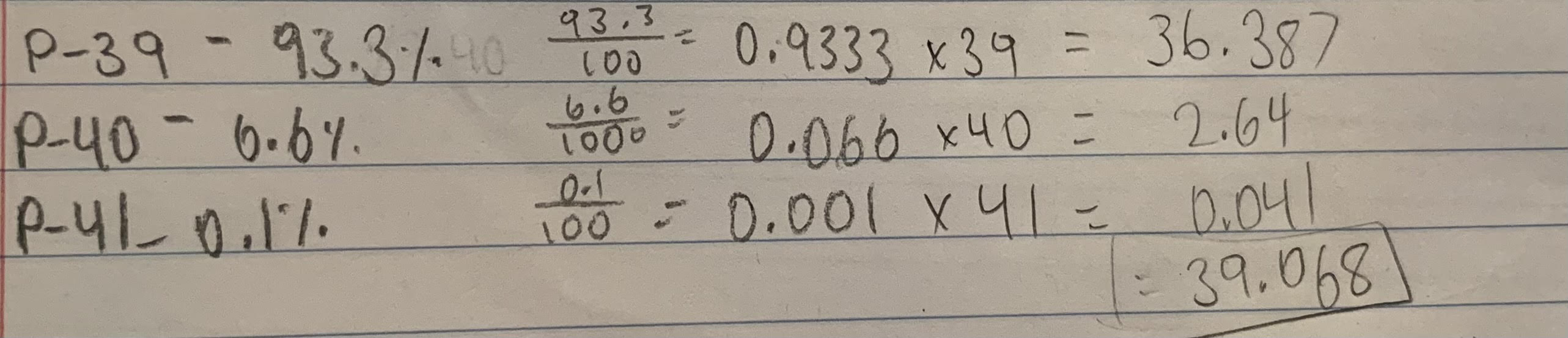
In this class, we progress from Bohr-Rutherford diagrams and developed the idea of CVR (Core, Valance, Radius) diagrams to explain properties of elements.
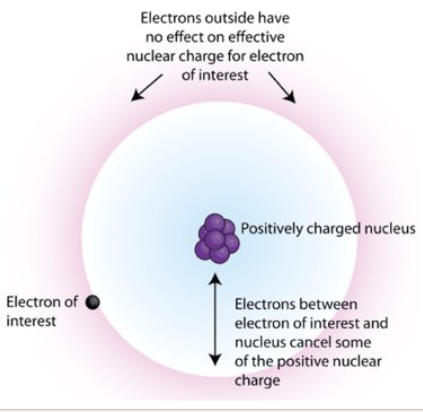
Explain the concept of Shielding that provides us with Zeff
The shielding effect is when core electronsn block off some of the core charge coming from the nucleus to the valance e-’s, making them only feel the effective core charge or Zeff. COre e-’s are the e-’s that are on the insider shells of an atom, not the valance or outermost shell. These core e-’s feel the full pull that the nucleus has on them, while also effectively shielding some of that pull so the valance e-’s only feel some of the attraction
What are the similarities and differences in CVR diagrams for elements in the same period and for elements in the same group.
Across a period, elements have their valance e'-’s in the same main she’ll and have the same core configurations. Zeff goes up by one and each element + the number for valance electrons also change by one
Down a group, elements have the same Zeff and valance structure, but have an increasing number of mainshells
State the trends in atomic radius on the periodic table and why the trends happen.
Down a group, elements have the same Zeff and valance structure, but have an increasing amount of mainshells. Across a period, elements ahve their valance electrons in the same mainshell as well as the same core configuration. Zeff goes up by one and each element and the number of valancne e also go up by one.
Across the table from left to right, Zeff is increasing, so while you are adding both e- and p+, each successive atom has one more p+ pulling on the e-. The added electron's are all in the same mainshell, and the increase in Zeff pulls the electron’s of that mainshell closer and closer to the nucleus, thus the atoms get smaller
Down a group, atoms get larger: This is because each step down a group means the addition of one more mainshell, so valance electrons occupy mainshells further away from the nucleus.
Metals and non-metals have differing properites largely because of how they hold their valance electrons. Explain and give examples.
EXPLAIN METALS
Lustre, Malleability, Ductility, Conductivity of heat and electricity
Metals: small core charges, less than half valance shell, large radii: electrons are less attracted to the core, unpaired and have more space for electrons to move from one to another, and valance e is further from the core. Structure is free
Lustre: Reflections result when free electrons free electrons at the surface of a metal interact with incoming light.
Conductivity: freely moving e- are attracted to ve+ and repelled by -ve electrodes, causing an electric current. As for heat, free e- can move thermal energy quickly
Malleability: atomic cores can easily slide into new positions through the sea of free electrons
Ductility: the ability of the metal nuclei to move pass each other without needing to break strong bonds is good
Metals and non-metals have differing properites largely because of how they hold their valance electrons. Explain and give examples.
EXPLAIN NON METALS
Non-metals: large core charges, at least half-filled valance shells, small radii: electrons are more attracted to the core, paired and not as free to move from atom to atom, close to core and overall strongly attracted and held. Structure is close, like a diamond
Lustre: Atoms in a non metal allow light to pass through, making it look transparent and not lustrous
Conductivity: the tightly bound electrons cannon flow between negative and positive electrodes. They also cannot transfer thermal energy
Malleability: The atoms in a non-metal like diamond break along specific planes because of the arrangement of the tightly bound electrons
T/F: Infrared energy is a form of electromagnetic energy whose short wavelengths can cause damage to skin
Ultraviolet
T/F: As the electron falls from the excited state in the diagram above, the energy emitted is in the form of heat energy
Light ener
FIB: Using a spectroscope, the unique set fo lines that can be used to identify an element is called an
Atomic Line Spectra
The smallest particle of an element that has all the properties of the element is
atom
Of the following, the particle that has the smallest mass is the
electron
The atomic number of an element is defined as the..
number of protons in the nucleus
The element found in the periodic table in Group 4 and Period 6 is
Hf, Hafnium
A metal in Group 15 is
phosphorus
Which of the following matches the group number and common name is INCORRECT
G2- Alkaline Earth Metals
G6 Lanthanides
G1- Alkali Metals
G17: Halogens
G18: Noble Gases
G6 Lanthanides
An atom of 14/6C has
6 protons and 8 neutrons
AN atomic mass unit (amu) is defined as the
one-twelveth the mass of a carbon atom
The atomic mass of Barium is due to the number of
protons and neutrons in the nucleus
The relative atomic mass of cholorine (35.452 amu) is a good indication that
Chlorine is a mixture of isotopes
The mass listed for each element in the periodic table is
the mass of the average number of neutrons in all of the isotopes of the element
The person given credit for developing the first modern periodic table is
Mendeelev
When atoms are arranged in order of increasing atomic mass, their chemical properities repeat at regular intervals. This statement is the definition of the
periodic law.
Other than helium, a full outer shell usually means
8 valance electrons
In Thompson’s raisin bun model,
the dough represents the positively charged sphere and the raisins represent the negatively charged electrons
The nuclear model of the atom was first proposed by
Rutherford
By conducting his gold foil experiment, Rutherford discovered
the nucleus
Each element produces a characteristic
line spectrum
The instrument that is used to separate light into its component colours is caled a
spectroscope
Bohr compared he electron in an atom to
Planets orbiting the sun (planetary model)
A region in space in which there is a high probability of finding and electron is the definition of an
orbital
Bohr’s model of the atom can be termed a “quantum” model of the atom because
each electron can posess only specific amounts of energy
Of which of the following is true about orbitals:
they are 3D probabilities
They can containing maximum of two electrons
they can exist as subshells within mainshells
all of the above
none of the above
all of the above
an electron that occupies a higher energy level than normal is said to be in
an excited state
valance electrons in an atom of chlorine feel an effective nuclear charge of
7+
two atoms are isotopes if they have
the same atomic number but different mass numbers
isotopes with unstable nuclei are also reffered to as
radioisotopes
atomic or ionic radius depend on…
nuclear charge
number of populated energy levels
number of valance electrons
The radius of an anion is usually BLANK than the neutral atom, while the radius of a cation is usually BLANK than the neutral atom
larger, smaller
Of which of the following elements require the least amount of energy to remove an electron from an atom to form an ion
O, He, K, H, Fr
Fr
Consider the equation X(g) + energy→X+ +e−
energy represents…
ionization energy
Electron affinity increases as you go left to right on the periodic table because
atomic radius decreases
Which of the following is the most reactive element
Helium, radon, hydrogen, oganesson, francium
francium
Consider the equation X(g) + e- →X- + energy
energy represents…
electron affinity
The structure fo the periodic table contains much information. IN terms of filling an atom with electrsons, each period going down represents a new BLANK that is further away from the nucleus
mainshell
In the quantum model of the atom, each subshell can contain a max of how many electrons
two
The noble gas neon contains eight valance electrons, which occupy the BLANK AND BLANK orbitals
2s 2p
Electronegativity comes from
ionization energies
electron affinity
atomic radius
Why do energy levels exist in atoms?
Electrons can only have specific quanta of energy
Electronegativity
the tendency of an atom to attract electrons from its neighbouring atom in a chemical bond/the measure of an atom’s ability to attract e-’s that are part of a chemical to itself
basically an atom’s ability to win a tug-of-war with other atoms over e, in a chem bond is the important part
Electron Affinity
energy change in an atom when a neutral atom gains an electron
X(g) + e- —> x(g) + energy
Metals lose e and form cations: Lower electron affinity bc they must be forced to gain e
Non-metasl gain e and form anions: Higher electron affinity bc they are more stable with more e
Ionization Energy
minimum energy needed t oremove an electron from an atom, how strong is an atom holding its valance electrons
X(g) + energy —> x+ + e-
Successive IE
the product of the first ionization is a ction with a 1+ charge, removing the next e will require more nergy ad each succesive ionization will require an increaing amount of energy
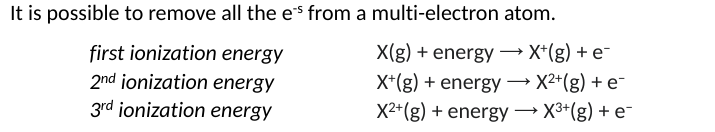
Periodic Law
When atoms are arranged in order of increasing atomic mass, their chemical properities repeat at regular intervals.
What are groups and periods, and the relationships between the elements within a group and within a period>
A group is a column of elemets going up and down on the periodic tale. ALl elements in one group have the same number of valance e-’s, and that number is the same as the group number.
A period is a row of elements going left and right, all elements in a period have their valance e-’s in the same shell and have the same number of shells, and that number is the same as the period nuber.
Provide the definition of ionization energy adn it’s interpretation as a chemical/mathmatical equation. State the trends in ionization energy across from left to right then down. Explain why the trends follow these patterns using concepts developed in the CVR model
Ionization energy is the minimum energy needed to remove an e= from an atom. The equation is X(g) + energy → x+(g) + e-
The trends in IE are that arcross the periodic table (L-R), IE increases and down a group, IE decreases.
IE increases from left to right because the Zeff increases and the radius decreases. This means valance electrons have a stronger attraction to its nucleus, making them closer and harder to remove.
IE decreases down a group because even though the Zeff is the same, the radius increases due to the increasing number of mainshells. The e-’s get farther from the pull of the nucleus, making them less attracted and easier to remove.
Using the concepts introduced with CVR diagrams and electronegativity, explain or use a T-table to explain why its predictable that metals tend to lose electrons and non metals tend to gain them.
It is predictable that metals will tend to lose e-’s because they have small Zeff’s, large radii, less than half filled valance shells and a small EN. Since they have small Zeff’s, the core doesn’t have a strong charge on the valance e-’s, making metals more likely to lose them. Their large radii makes it so they are far from the nucleus and therefore less attracted as well. Since metal valance shell’s are less than half filled, they are unpaired which makes it easier to go from atom to atom. Finally, their small EN means they have a harder time attracting electrons.
As for non-metals, it is predictable that they will gain electrons because of their large Zeff’s, small radii, more than half filled valance shells and larger EN. Their large Zeffs mean that the core has a stronger charge on the valance e-’s, making them more attracted. Their small radii means that the valance e-’s are closer to the core and are more attracted as well. The greater than half filled valance shells mean that they can be paired which makes it harder for them to move from atom to atom. Finally, their large EN means they can attract more electrons.
Comparing the properties of metals vs non metals, differenciate bewteen free verese bound electrons, ad then use these concepts (lustre, malleable, ductile, conduct) to compare them. You only need to address TWO of the properties.
Metals:
Metals have free electrons due to their small Zeff, less than half filled valance shell and large radii, causing the electrons to be less attracted to the core, unpaired and have more space to move from one to the otehr, and make valance electrons further from the core. Metals can conduct electricity because freely moving e- are repelled by the -ve electrode and attracted to the +ve electrode, setting up an electric current. Metals are also malleable because the atomic cores can slide int new positions through the sea of electrons, causing them to be flattened or shapeable
Non Metals
Non metals have tightly bound electrons due to their large core charges, at least half filled valance shells and small radii, causing e- to be highly attracted to the core, paired and not as free to move, and close to the core. Non metals and their tightly bound elections cannot flow between negative and positive electrodes, making them bad conductors. Non-metals also breaks long specfici planes due to the arrangement of the tightly bound electrons (ie. diamond like structure in carbon)
Explain how Bohr’s model builds on but is different from Rutherford’s
Bohr’s model has the same basics as Rutherford’s model. BOth of them have nuclei and electrons. Howeer, in Rutherford’s model of the atom, e-’s were zipping around everywhere, similar to bee’s in a beehive. Bohr quantized the and organized the e-’s into orbits. This means e-’s can only exist in allowable orbits, not in between or zipping around. They also could only have specific amounts of energy in their specific orbits. Bohr’s model can be compared to planets orbitting the sun, for it is organized, unlike bees in a beehive like Rutherford’s model.