topic 3 - particle model of matter
1/47
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
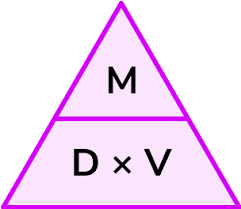
state the equation linking density, mass and volume
density/ρ (kg/m³) = mass/m (kg) / volume/v (m³)
state what the particle model can be used to explain
the different states of matter
differences in density
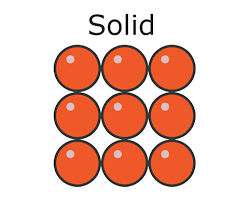
explain how to draw simple diagram of a solid matter
draw 9, equally-sized circles
all the circles should be touching 2 other circles
they should be arranged in a regular 3×3 pattern
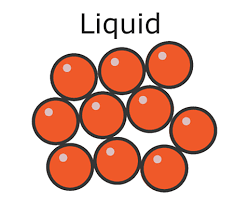
explain how to draw simple diagram of a liquid matter
draw 9, equally-sized circles
all circles should be touching at least one other circle
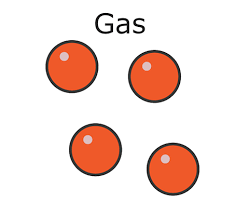
explain how to draw simple diagram of a gas matter
draw 4, equally-sized circles
all circles should have no contact with other circles
explain what the density of solid matter is based on particle arrangement
tightly packed arrangement leads to solids being high density matter
due to the large amount of mass confined in a small volume
explain what the density of liquid matter is based on particle arrangement
moderately close arrangement leads to liquids being moderate density matter
due to the moderate amount of mass confined in a given volume
explain what the density of gas matter is based on particle arrangement
widely spaced arrangement leads gases being low density matter
due to small amount of mass spread out over a large volume
explain the method of determining the density of regular objects
measure the height, width and length of the object using a ruler
multiply these together to find the volume of the object
use an electric balance to measure the mass of the object
input these values into the equation d = m/v to find the density of the object
explain the method of determining the density of irregular objects
measure out a fixed volume of water into a measuring cylinder
use an electric balance to measure the mass of the object
gently submerge the object into the water fully and record the new volume of water
minus the initial volume of water from the final volume to find the volume of the water displaced
volume of water displaced is the volume of the object
use the volume and mass results in the equation d = m/v to find the density of the object
describe how, when substances change state, mass is conserved
when the substance changes state, the particles in the matter rearrange and join back together to form a new substance
but the number of atoms stays the same
conserving the mass of the matter
state what kind of change changes of state are
physical changes
explain how physical changes differ from chemical changes
physical changes - the material can recover their original properties if the change is reversed
chemical changes - the material can’t recover their original properties if the change is reversed
explain why a material can’t recover their original properties if chemical changes are reversed
when chemical changes occur, new products are formed
these products have different chemical compositions to the reactants
state how internal energy is stored inside a system
by the particles that make up the system
state what internal energy is made up of
total kinetic energy + potential energy of all particles that make up the system
explain how heating affects energy stored within a system
heating increases the kinetic energy of the particles within the system
this either raises the temperature of the system
or produces a change of state
causing an increase in the system’s internal energy
state what happens if the temperature of a system increases
the kinetic energy of the particles in the system will increase
state what the temperature increase of a system is dependent on
mass of the substance heated
type of material
energy input to the system
state the equation linking energy change, mass, specific heat capacity and temperature change
energy change (J) = mass (kg) x specific heat capacity (J/kg°C) x temperature change
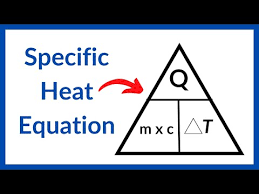
state the symbol equation linking energy change, mass, specific heat capacity and temperature change
ΔE (J) = m (kg) x c (J/kg°C) x Δθ (°C)
explain what specific heat capacity of a substance is
amount of energy required
to raise the temperature
of one kg of a substance
by one degree celsius
state what the energy needed for a substance to change state is called
specific latent heat
state what happens to the internal energy and temperature of a system when the energy supplied changes
internal energy changes
temperature remains the same
explain what specific latent heat of a substance is
amount of energy required
to change the state
of one kg of a substance
with no change in temperature
state the equation linking energy for change of state, mass and specific latent heat
energy for change of state (ΔE)= mass (kg) x specific latent heat (J/kg)
state the symbol equation linking energy for change of state, mass and specific latent heat
ΔE = m x L
state what the specific latent heat of fusion is
change of state from solid to liquid
state what the specific latent heat of vaporisation is
change of state from liquid to vapour
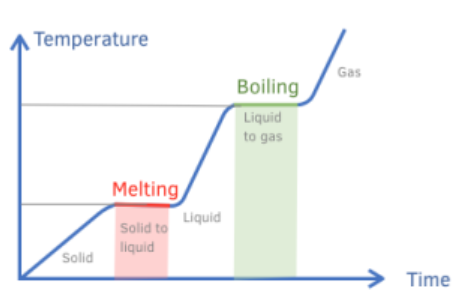
explain how to interpret a heating graph
horizontal line is when a substance is undergoing a change of state
the temperature remains constant during the horizontal lines
positive diagonal line is substance being heated
the temperature increases during the positive diagonal lines
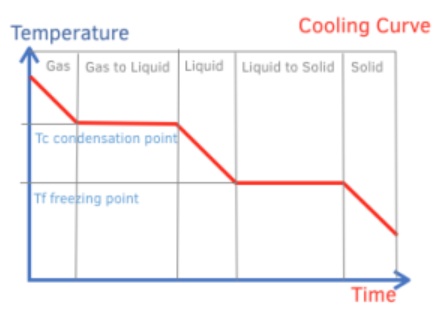
explain how to interpret a cooling graph
horizontal line is when a substance is undergoing a change of state
the temperature remains constant during the horizontal lines
negative diagonal line is substance being cooled
the temperature decreases during the negative diagonal lines
explain the differences between specific heat capacity and specific latent heat
specific heat capacity - change in temperature
specific latent heat - change in state
state the type of motion of gas molecules
random motion
state what the temperature of a gas is related to
the average kinetic energy of the gas molecules
state how changes in temperature of gases affects the pressure exerted by the gas
increasing temperature increases the kinetic energy of the gas molecules in a constant volume
which increases the frequency of successful collisions between gas molecules
which generates force
force is directly proportional to pressure
thus causing an increase in pressure
explain how the motion of gas molecules is related to temperature
temperature increase increases kinetic energy of molecules
gases move in random motion
due to higher kinetic energy than other states
causing more collision between molecules
and more force generated by more frequent collisions
at constant volume
explain how the motion of gas molecules is related to pressure
gases move in random motion
due to higher kinetic energy than other states
causing more collision between molecules
and more force generated by more frequent collisions
force is directly proportional to pressure
so gas pressure is increased at constant volume
explain qualitatively the relation between temperature of gas and its pressure at constant volume
as volume of container with a gas remains constant
the pressure of a gas increases
as its temperature increases
due to increasing kinetic energy of molecules
causing excess collisions between molecules and walls of container
creating excess force
force is directly proportional to pressure
state what allows a gas to be compressed or expanded
compressed - decrease in pressure
expanded - increase in pressure
explain what kind of force gas produces on the wall of the container holding the gas
net force
perpendicular to walls of container
explain how to use the particle model to explain how increasing volume of which gas is contained can lead to a decrease in pressure #
pressure and area/volume are inversely proportional
as pressure increases when there are more collision between gas molecules
which generates excess force
as volume increases, the space that the gas molecules have to move increases
and space between gas molecules increases
decreasing the chance of collisions between molecules and the walls of the container
state the equation that links pressure, volume and constant temperature
constant temperature (°C) = volume (m³) x pressure (Pa)
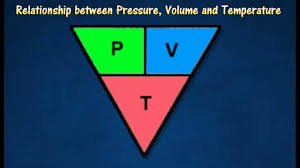
state the symbol equation that links pressure, volume and constant temperature
constant (°C) = V (m³) x p (Pa)
state the equation that links initial volume and pressure to final volume and pressure
pressure₁ (Pa) x volume₁ (m³) = pressure₂ (Pa) x volume₂ (m³)
state the symbol equation that links initial volume and pressure to final volume and pressure
p₁ (Pa) x V₁ (m³) = p₂ (Pa) x V₂ (m³)
state what work is in relation to force
work is the transfer of force
state what happens when work is done on a gas
internal energy increases
causing an increases in gas temperature
explain how doing work on an enclosed system leads to an increase in temperature of the gas - bicycle pump
a bicycle pump pumps oxygen into a tire
this will increase the number of gas molecules in the tire whilst the area remains the same
this will increase the frequency of successful collisions between molecules and the walls of the tire
the force of the collisions causes work to be done
which increases the average kinetic energy of the molecules
which increases the temperature of the oxygen through energy transfer to the thermal store of the oxygen