PHR 938 - Block 2 kaitlyn
1/113
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
114 Terms
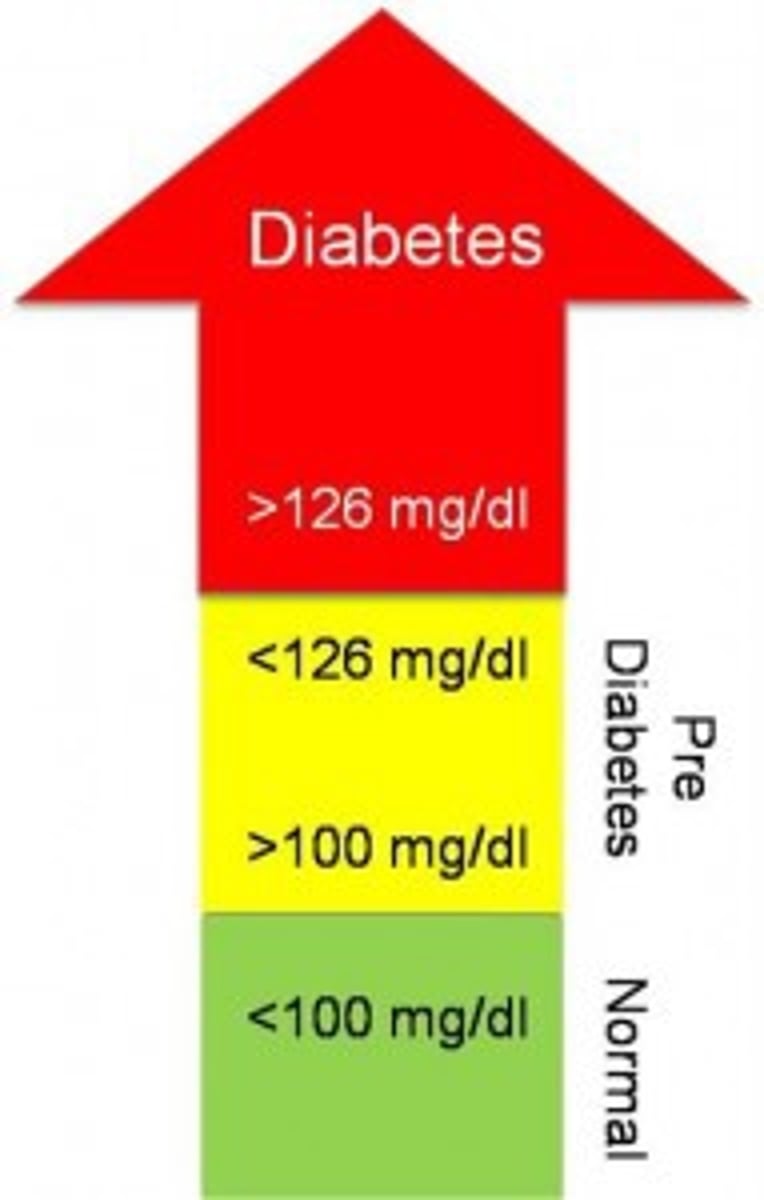
prediabetes
higher than normal blood sugars, but not high enough to diagnose with diabetes
- high prevalence, most unaware that they have it
- very high risk for developing diabetes and cardiovascular disease
do you always progress to diabetes if you have prediabetes?
not always
- 25% progress to diabetes
- 50% remain in abnormal glycemic state
- 25% revert to normal glycemic state
- people with both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are at double the risk of developing diabetes
prediabetes lifestyle modifications
- aim for 5-7% reduction in weight to reduce risk
- nutrition therapy
- physical activity
- smoking cessation
- psychosocial care
- sleep health
- diabetes self-management education and support (DSMES)
prediabetes medications
there is no FDA approved agent with a specific indication for T2DM prevention
options (off-label):
- metformin, a-glucosidase inhibitors, GLP-1 agonists, TZDs, insulin, valsartan, testosterone
- weight loss medications (orlistat, phentermine, GLP-1 RAs)
is metformin effective in prediabetes?
not as effective (1/2) as lifestyle modifications
- it is more effective in younger pts, obese pts, higher FPG, higher A1C, and people with previous gestational diabetes
National Diabetes Prevention Program
- year long dietary and lifestyle program focused on long-term results
- certified centers around the country
- professional and social support
- cost varies by center. insurance often will cover the program
criteria:
18+, overweight, not diagnosed with T1/T2, and not currently pregnant AND meet 1 of these:
- diagnosed with prediabetes
- previous gestational diabetes
- high risk on prediabetes test
general nutrition guidelines
- emphasize fruits, vegetables, whole grains, lean protein, low-fat or non-fat dairy
- limit saturated fat, trans fatty acids, cholesterol, sodium, and added sugars
- achieve a balance of carbs throughout the day
- consume concentrated sweets in moderation
- include proteins and fats as well as carbs at meals and snacks
physical activity requirements
children:
- 60+ min/day of moderate-vigorous intensity aerobic activity
- vigorous muscle-strengthening and bone-strengthening activities 3 days/week
most adults with T1 and T2 diabetes:
- 150 of moderate-vigorous intensity aerobic activity
immunization recommendations
- pneumococcal
> PCV20: get once
- hep B series (under 60 or at risk)
- Tdap
- zoster
- HPV (<26 or 27-45 if risk factors)
- COVID-19
- flu (annual)
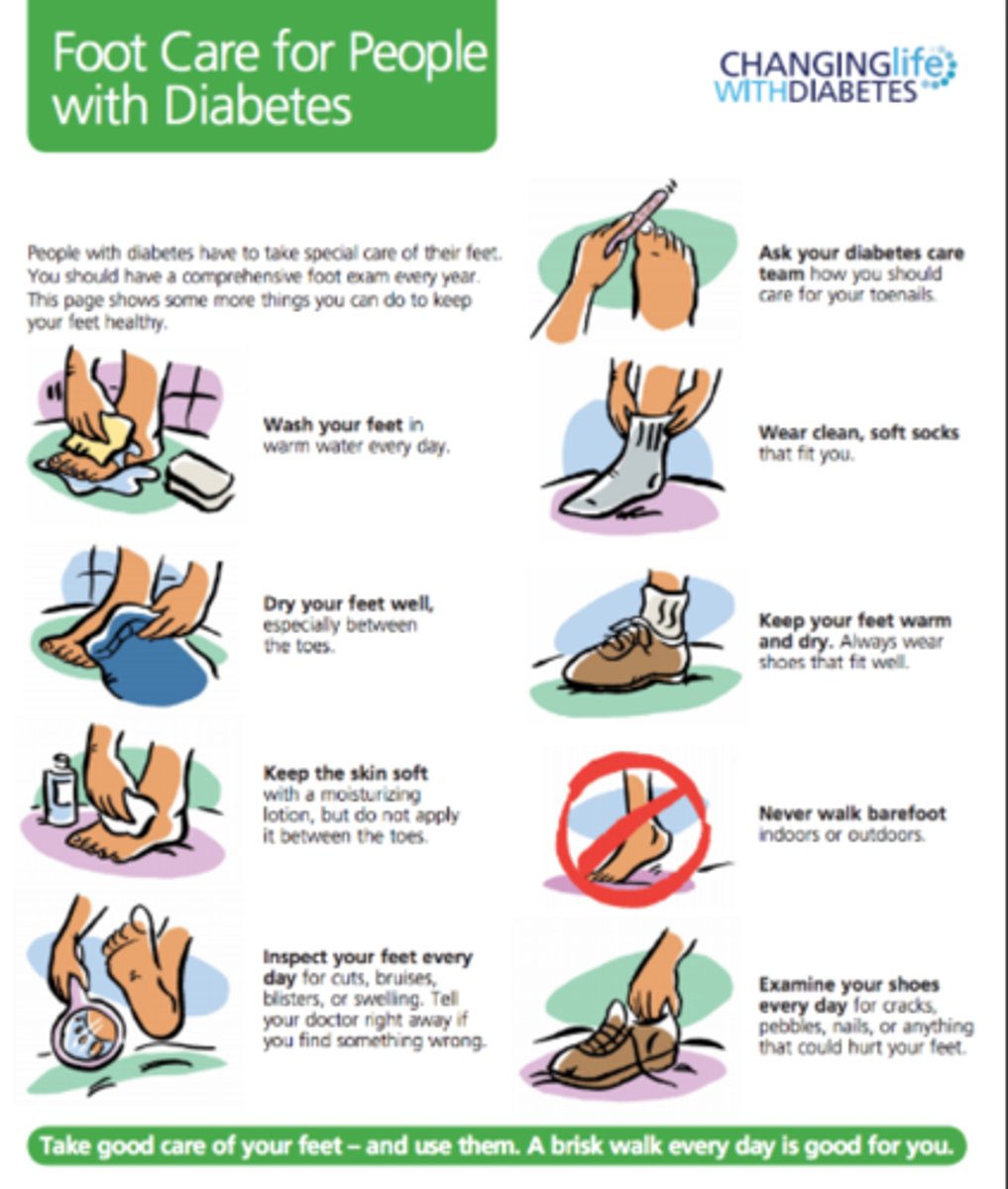
foot care
- comprehensive foot exam annually to check for ulcers/ loss of sensation
- if previous foot issues: foot exam at EVERY visit
- examine: skin, deformities, neuro assessment, vascular assessment
glycemic goals in the hospital
140-180
when to initiate insulin in the hospital
when pt has persistent hyperglycemic (2+ readings) of 180+ mg/dL
- stricter goals if they can be achieved without hypoglycemia
who has higher glucose goals in the hospital?
- terminally ill
- pts with severe comborbidities
- inpatient care where frequent BG monitoring/ close nursing supervision isn't possible
factors to account for when dosing insulin in the hospital
- type of diet ordered (PO, NPO, TPN, tube feed)
- medications causing hyperglycemia
- weight-based dosing
- home glycemic regimens
- degree of stress response
- kidney function
how is BG monitored in the hospital?
- PO pts: BG check before each meal and at bedtime
- NPO pts: BG check every 6 hours
- pts on IV insulin: BG check every 30 mins - 2 hours
insulin dosing: non-critically ill pts
- if on insulin at home: use TDD upon admission -> use 70-80% of TDD while hospitalized
- if not on insulin at home: dose by body weight
> 0.4 units/kg (normal weight)
- use SQ rapid or short acting AFTER meals
> or give every 6 hours if no meals are given
rapid insulin
Lispro (Humalog)
Aspart (Novolog)
Glulisine (Apidra)
normal insulin (regular)
Humulin R
Novolin R
basal insulin
Glargine (Lantus, Toujeo, Basaglar, Semglee)
how to split basal and rapid units? (PO pts)
basal dose = 50% of TDD
rapid dose = 50% of TDD
+ rapid-acting correction insulin
how to split basal and regular units? (tube/ TPN pts)
basal dose = 30% of TDD
regular insulin bolus = 70% of TDD - give every 6 hours
+ regular insulin correction
how to split basal and rapid units? (NPO pts)
basal dose = 50% of TDD
HOLD bolus until nutrition is established
+ regular insulin correction
insulin dosing: critically ill pts
- continuous IV insulin
- initiate when glucose is 180+ mg/dL
- maintain 140-180 mg/dL
how to transition from IV to SQ insulin
- administer 70-80% of IV TDD
- divide basal and bolus doses 50/50
- give a dose of SQ basal insulin 2 hours before d/c IV insulin
endocrine system
glands that control many of the body's activities by producing hormones
- affects metabolism, growth, water/electrolyte balance, reproduction, and behavior
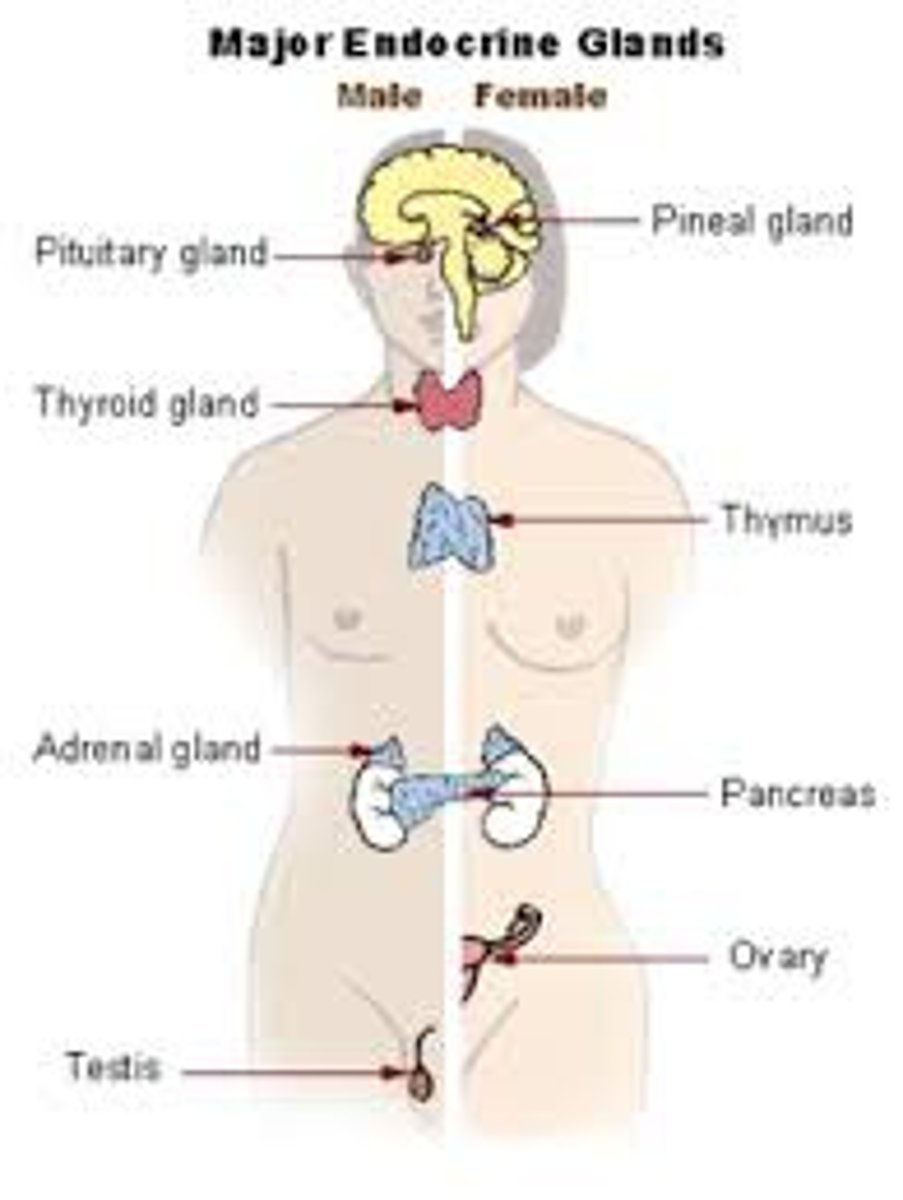
3 components of the endocrine system
1. glands - ductless
- not anatomically connected to the target site
2. hormones - several classes with differing characteristics
3. target organs - contain hormone specific receptors
- hormone-receptor complex initiates steps that produce biological effects
hormone
chemical messengers, mostly those manufactured by the endocrine glands, that are produced in one tissue and affect another
3 classes of hormones
1. steroid
2. tyrosine derivatives
3. peptides and proteins
- glycoproteins
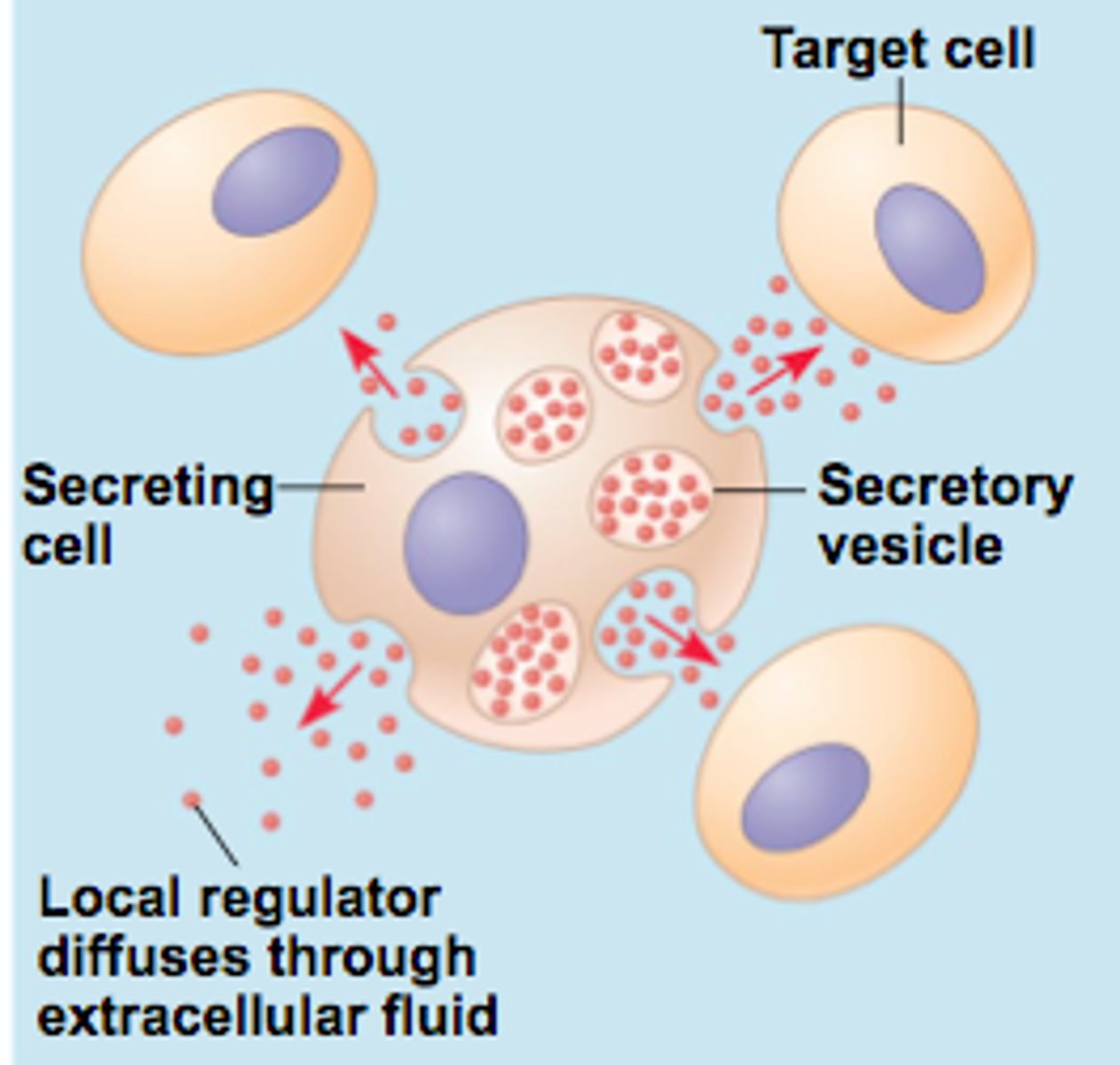
5 ways hormones get to their site of action
1. endocrine:
released by gland -> circulation -> target organ
(insulin, thyroid)
2. neuroendocrine:
secreted by neuron -> circulation -> target organ
(growth hormone, ADH, oxytocin)
3. paracrine:
secreted by cells -> extracellular fluid -> affects nearby cells of a different type
(FSH, GnRH)
4. autocrine:
secreted by cells -> extracellular fluid -> affects function of same type of cell
(prostaglandins, IL-1)
5. intracrine:
produced by and acts within a single cell
pulsatile release of hormones
some hormones (esp hypothalamic → pituitary) are secreted in bursts (pulses) rather than constant levels
- prevents desensitization/down-regulation of receptors.
-ex: GnRH must be pulsatile to stimulate LH/FSH release
- allows body to fine-tune physiologic responses (growth, reproduction, stress).
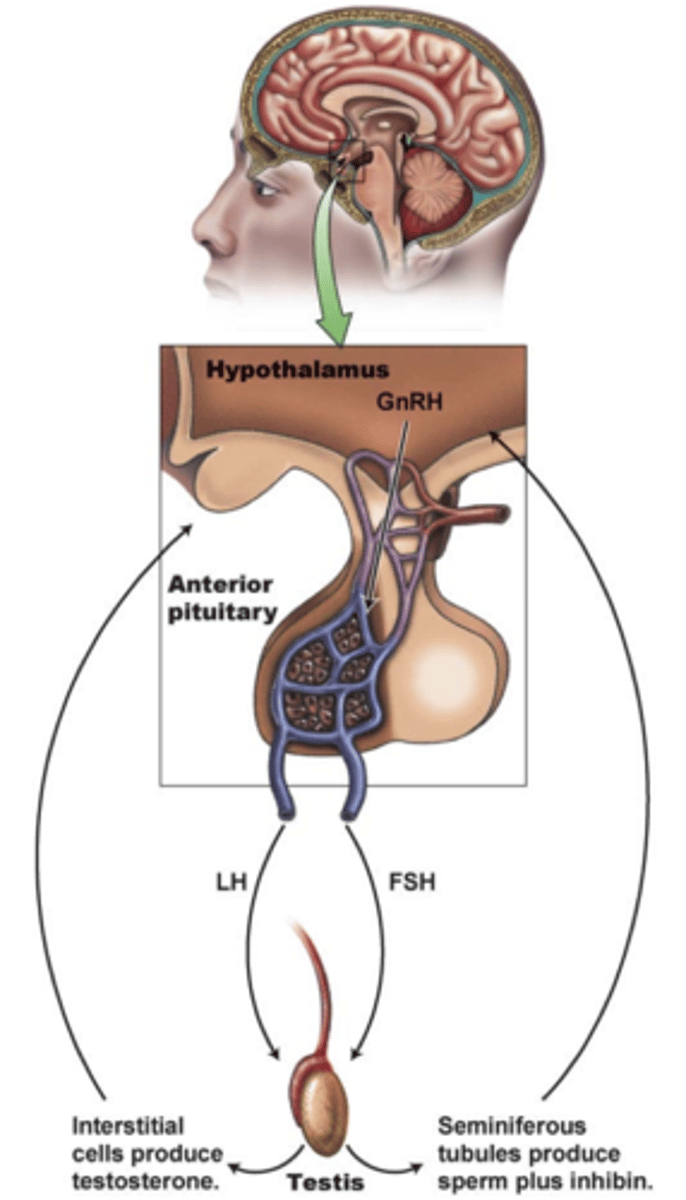
3 ways hormone secretion is controlled
1. negative feedback
- biological response suppresses hormone secretion
2. positive feedback
- needed to gather momentum for a specific outcome
(childbirth, ovulation)
3. circadian rhythms
- diurnal (daily, sleep/wake) or infradian (longer, menstrual cycle)
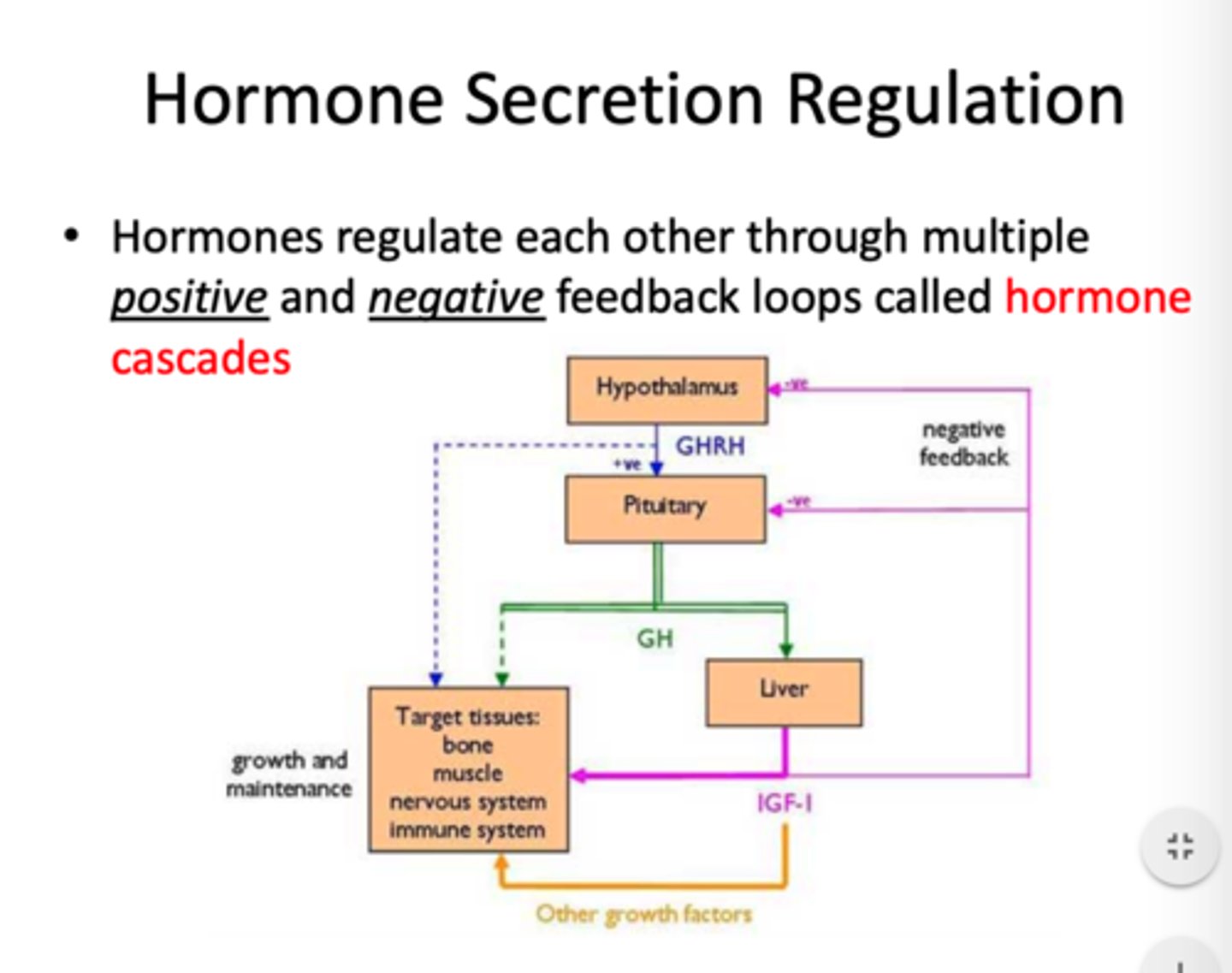
negative feedback system
hypothalamus produces releasing factors that stimulate the pituitary gland
↓
trophic hormones produced that stimulate target organs
↓
hormone production provides negative feedback to hypothalamus
↓
pituitary decreases releasing factor and trophic hormone production
- ↓ hormone levels = negative feedback is released and releasing factor production increases again
3 types of endocrine disorders
1. hormone deficiency
- too little
- due to: destruction of target organ cells, autoimmune response, genetic factors, lack of precursors/ enzymes
- ex: type 1 diabetes = no insulin production
2. hormone excess
- too much
- due to: tumors that overproduce, receptor overstimulation, or antibodies that trigger receptor action
- ex: hyperinsulinemia
3. hormone resistance
- doesn't work
- due to: desensitization of target cells, receptor fails to signal machinery
- ex: type 2 diabetes
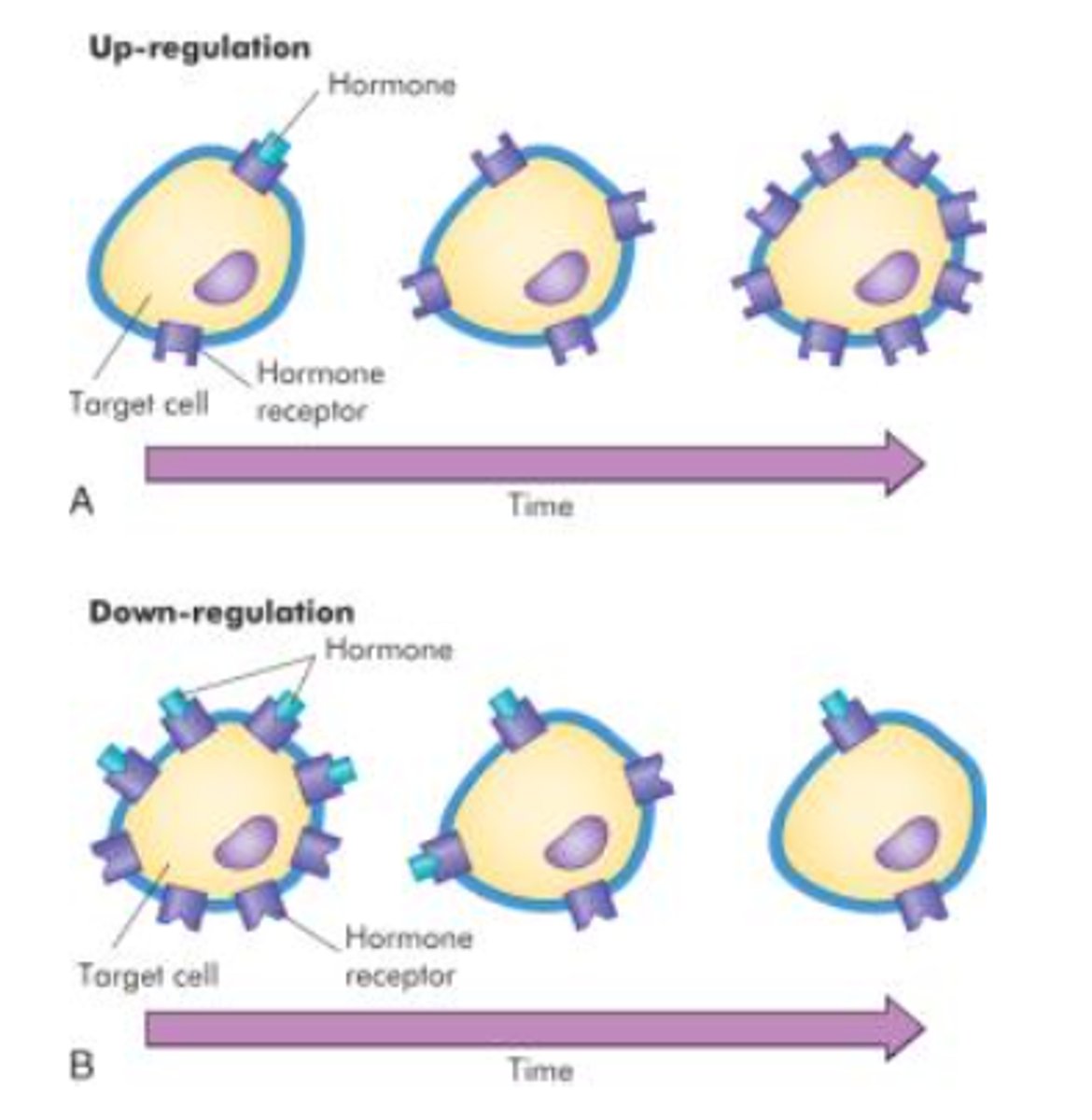
how are hormone receptors regulated?
up-regulation: greater production of receptors or associated proteins
- the target cell is MORE responsive to the hormone
down-regulation: inactivation, destruction, decreased production of receptors or associated proteins
- target cell is LESS responsive to the hormone
these work in tandem to maintain steady levels
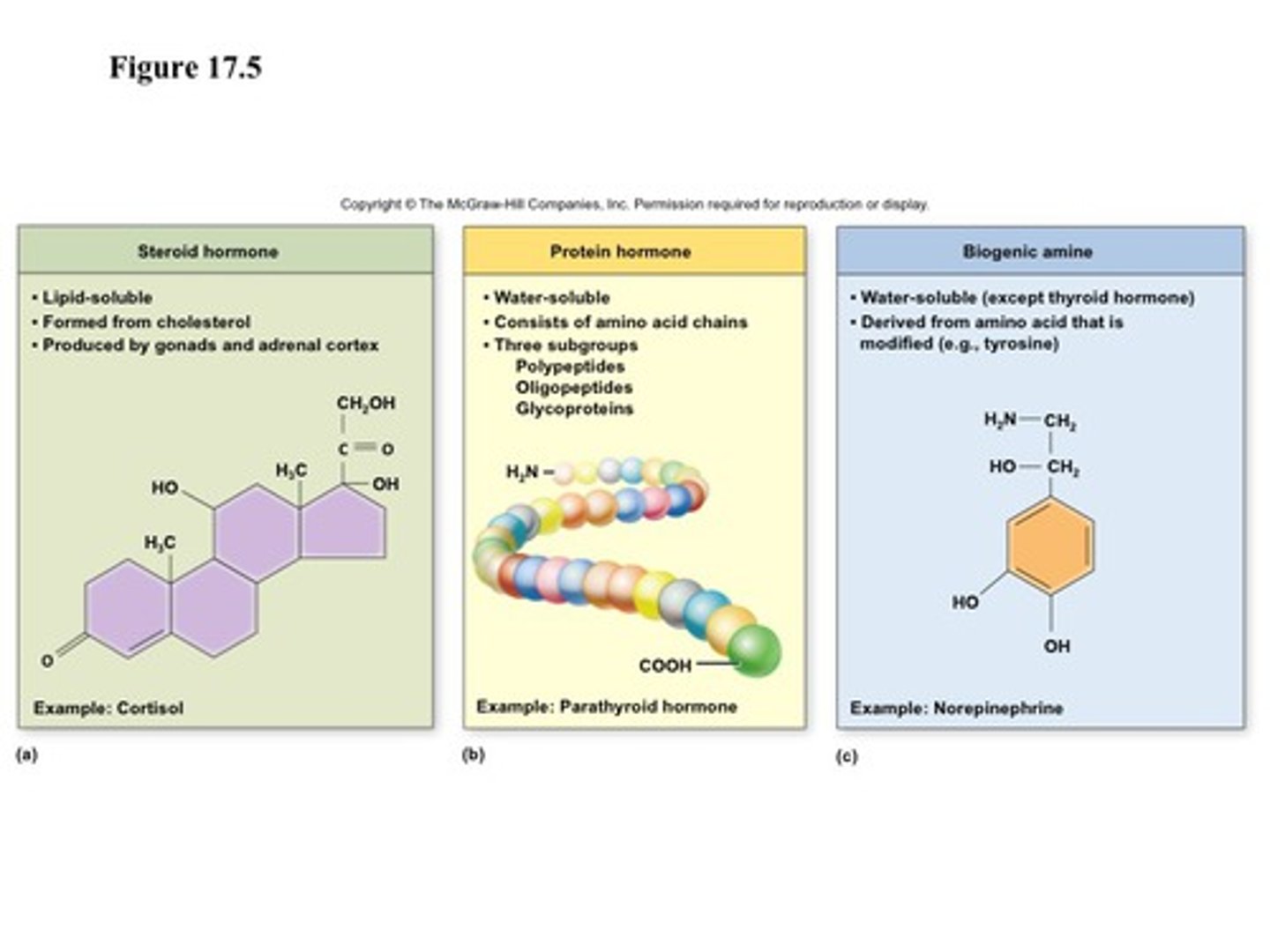
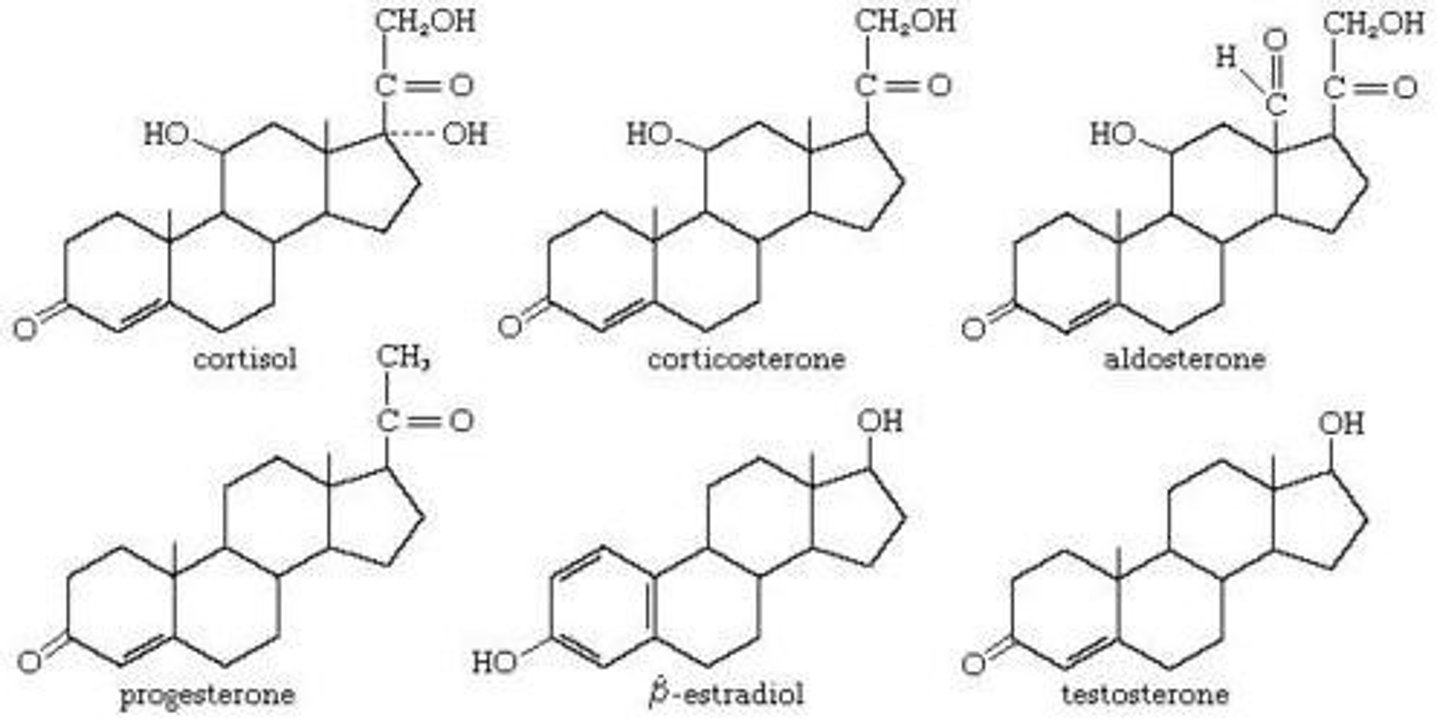
steroid
hormone class
- derived from: cholesterol
- synthesized in: adrenal cortex, gonads, placenta
- stored: not stored
- released: upon synthesis by diffusion out of the cell
- solubility: lipid soluble, bound to binding proteins in plasma (not free)
- t1/2: 20 min-24 hrs (binding proteins extend)
- receptor: intracellular (cytosolic or nuclear)
> crosses plasma membrane
- ex: reproductive hormones, cortisol, vit D
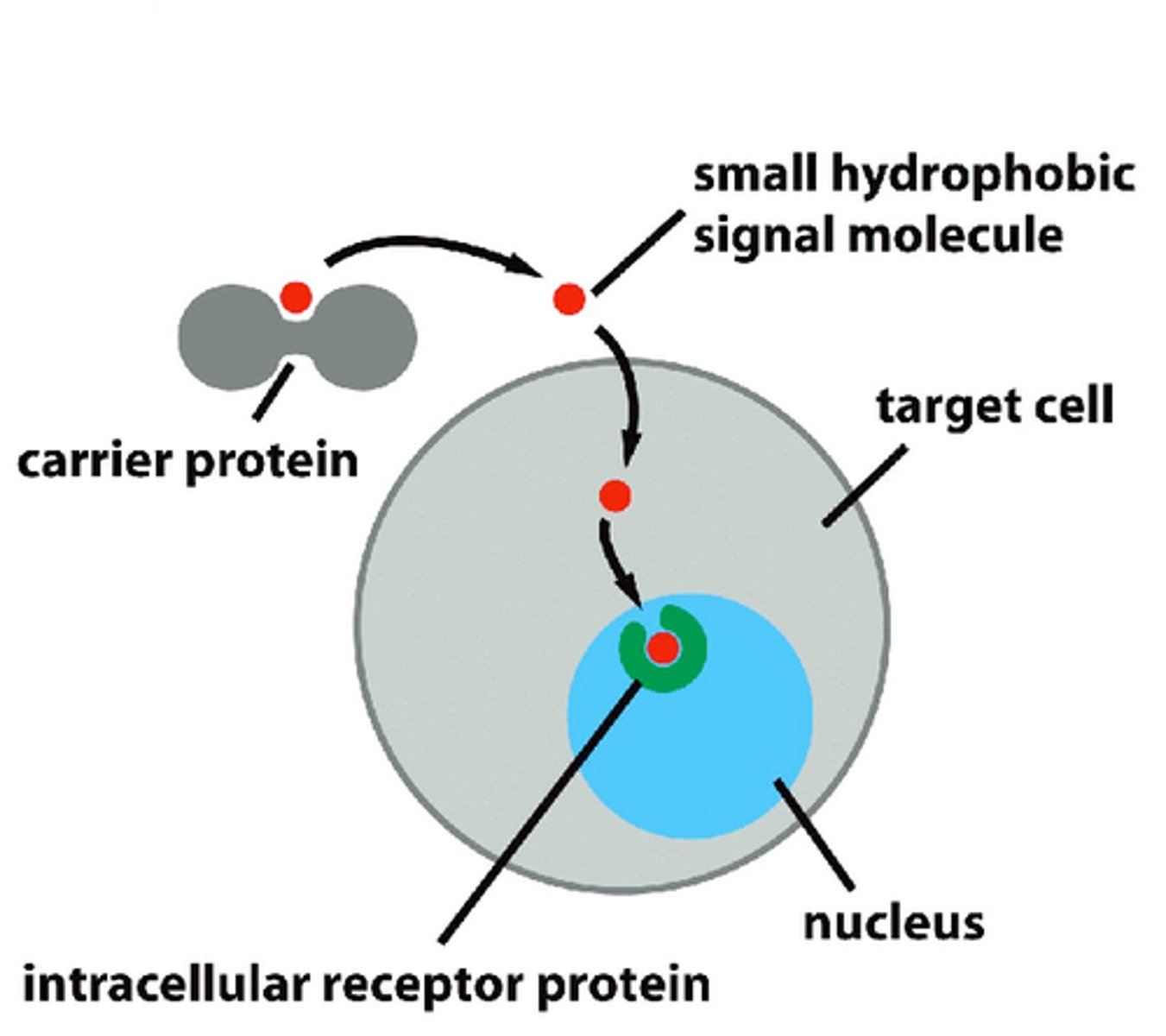
intracellular receptors
receptors located inside the cell rather than on its cell membrane
proteins/ peptides
hormone class
- synthesized: as inactive pre-prohormones
> cleaved to prohormones before storage (active)
- stored: in secretory granules in cytoplasms
- released: into interstitial fluid or circulation
- solubility: water soluble, no binding proteins
- t1/2: 4 min-2 hrs
- receptor: membrane bound GCPR
> does not cross plasma membrane
- ex: insulin, glucagom, GLP-1, growth hormones
glycoproteins
hormone class (subunit of proteins)
- contain 2 subunits: a common a subunit and a distinct B subunit
- ex: TSH, LH, FSH, hCG
- 200+ amino acids
- glycosylation -> alpha carbohydrate side chain added
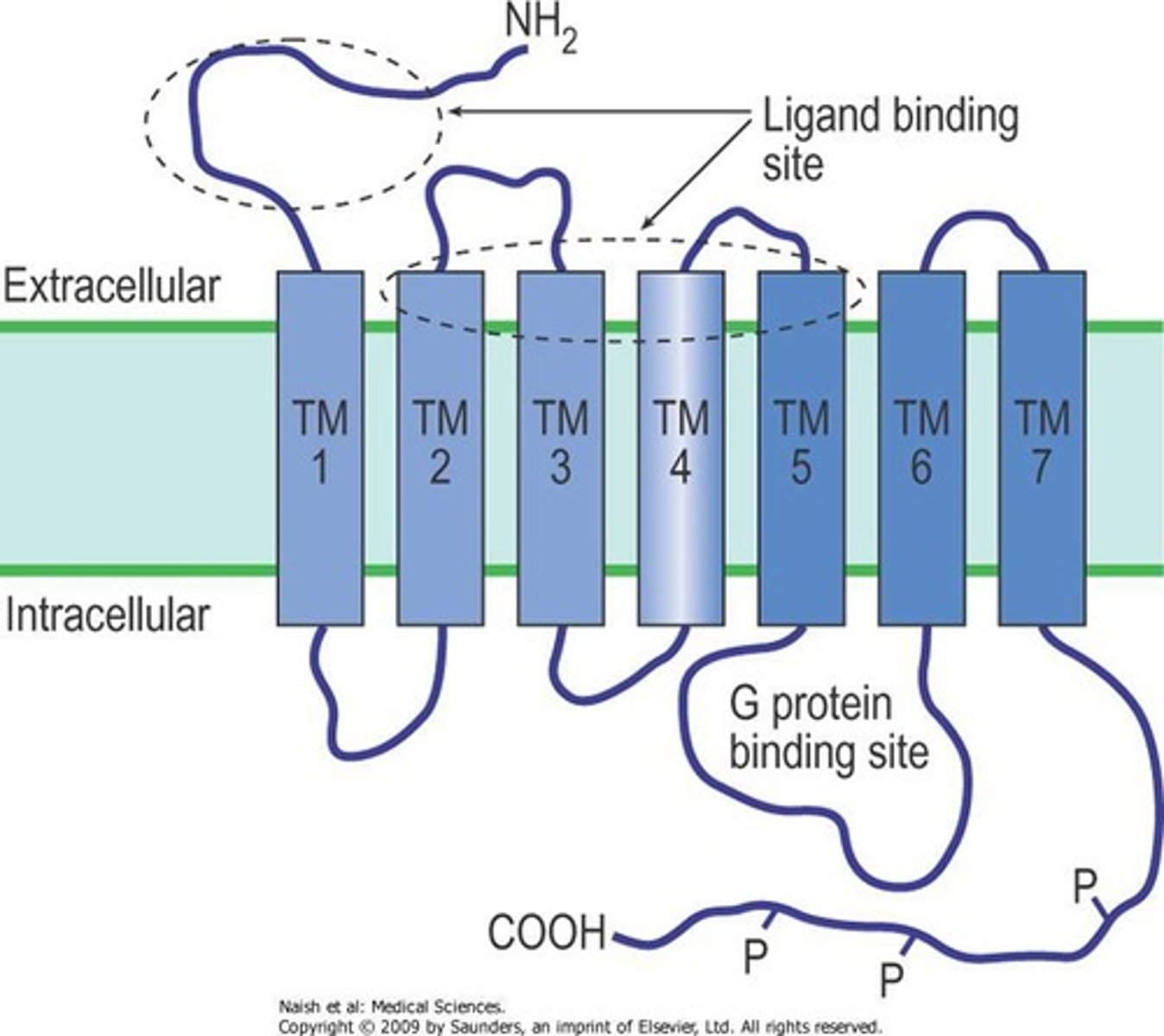
G-protein coupled receptors (GPCR)
a cell-surface transmembrane receptor that works with the help of a G protein
- contain 7 transmembrane spanning segments
- the N terminus faces the extracellular space and contains the ligand-binding domain
- the C terminus is on the cytosolic side and is associated with 2nd messenger generating G-proteins
- 2nd messenger systems mediate a series of steps leading to the final hormonal action
- effects can be inhibitory or stimulatory
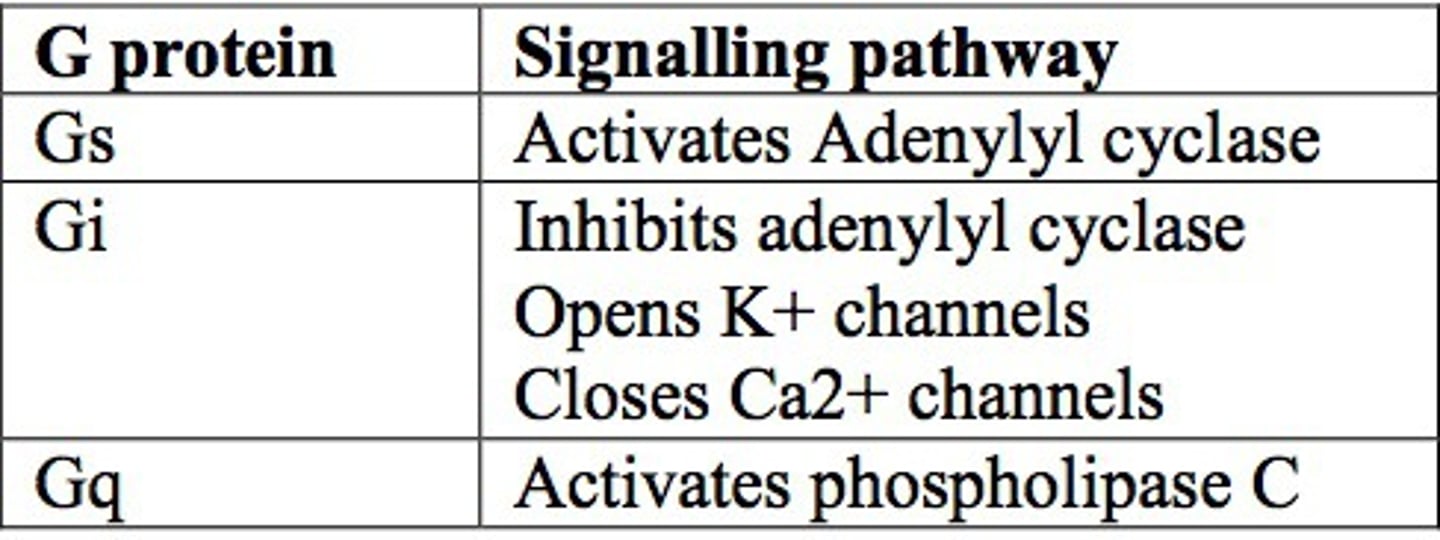
Gs, Gi, and Gq 2nd messenger system
Gs: α-subunit activated adenylate cyclase -> increases cAMP production
Gi: α-subunit inhibits adenylate cyclase -> decreases cAMP production
Gq: α-subunit activates PLCβ -> increased IP3 -> increases cytoplasmic Ca
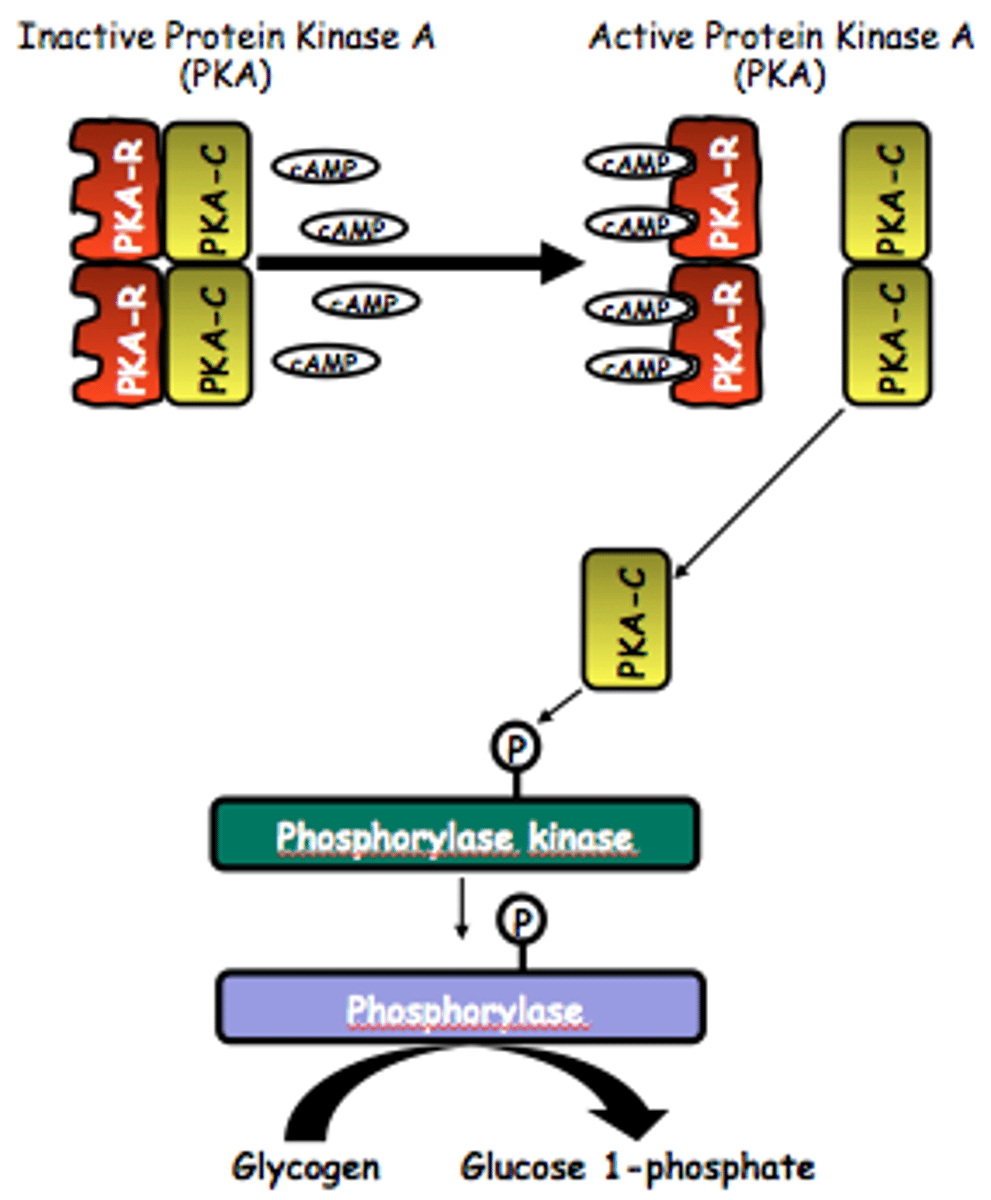
Gi/Gs pathway
adenyl cyclase (AC) stimulation
↓
formation of cAMP
↓
activates protein kinase A (PKA)
↓
phosphorylates proteins that mediate hormonal activity
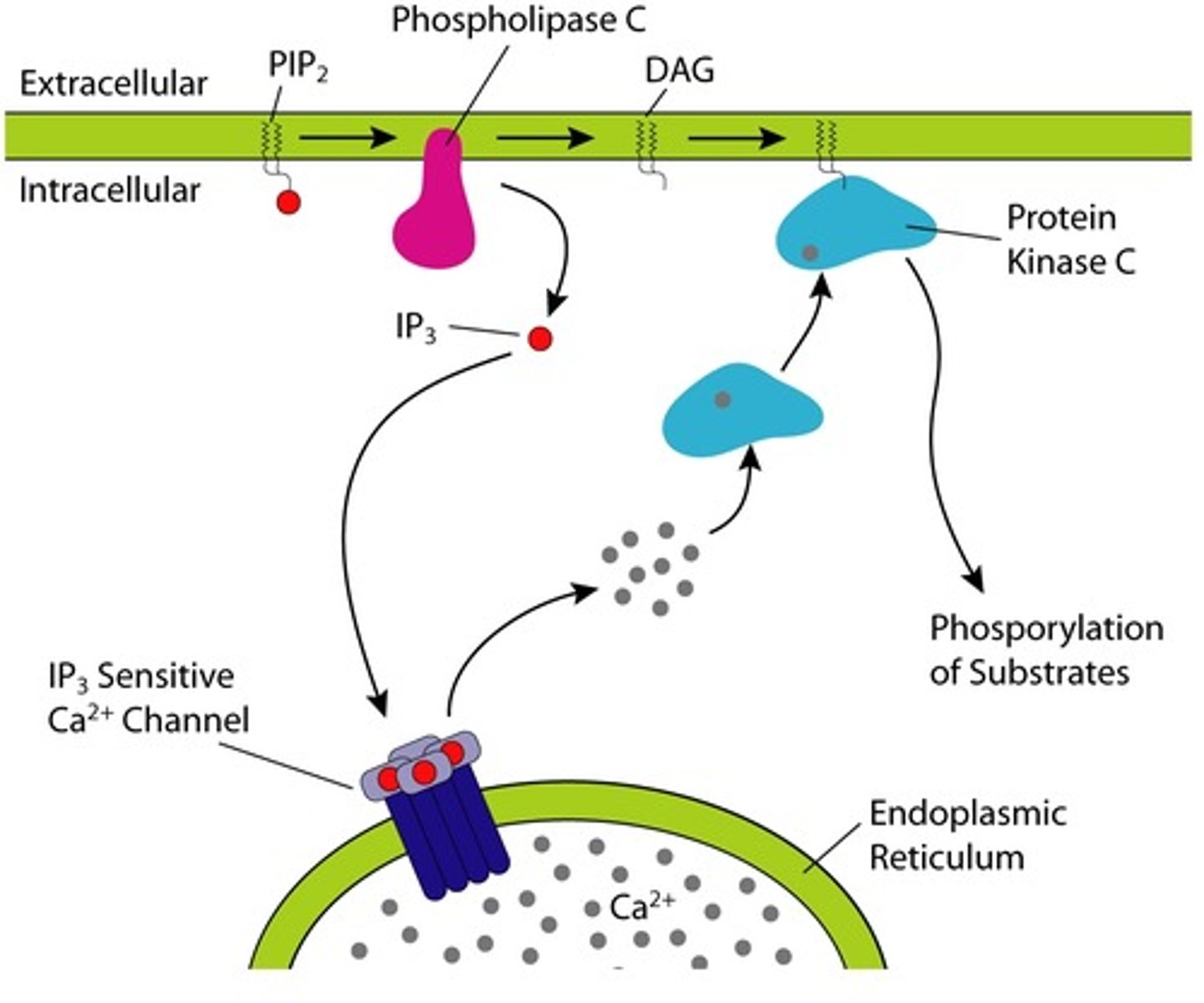
Gq pathway
phospholipase C (PLC) activation
↓
phospholipids split into diacylglycerol (DAG) and inositol triphosphate (IP3)
↓
activation of protein kinase C (PKC)
↓
alters activity of enzymes that mediate hormonal activity
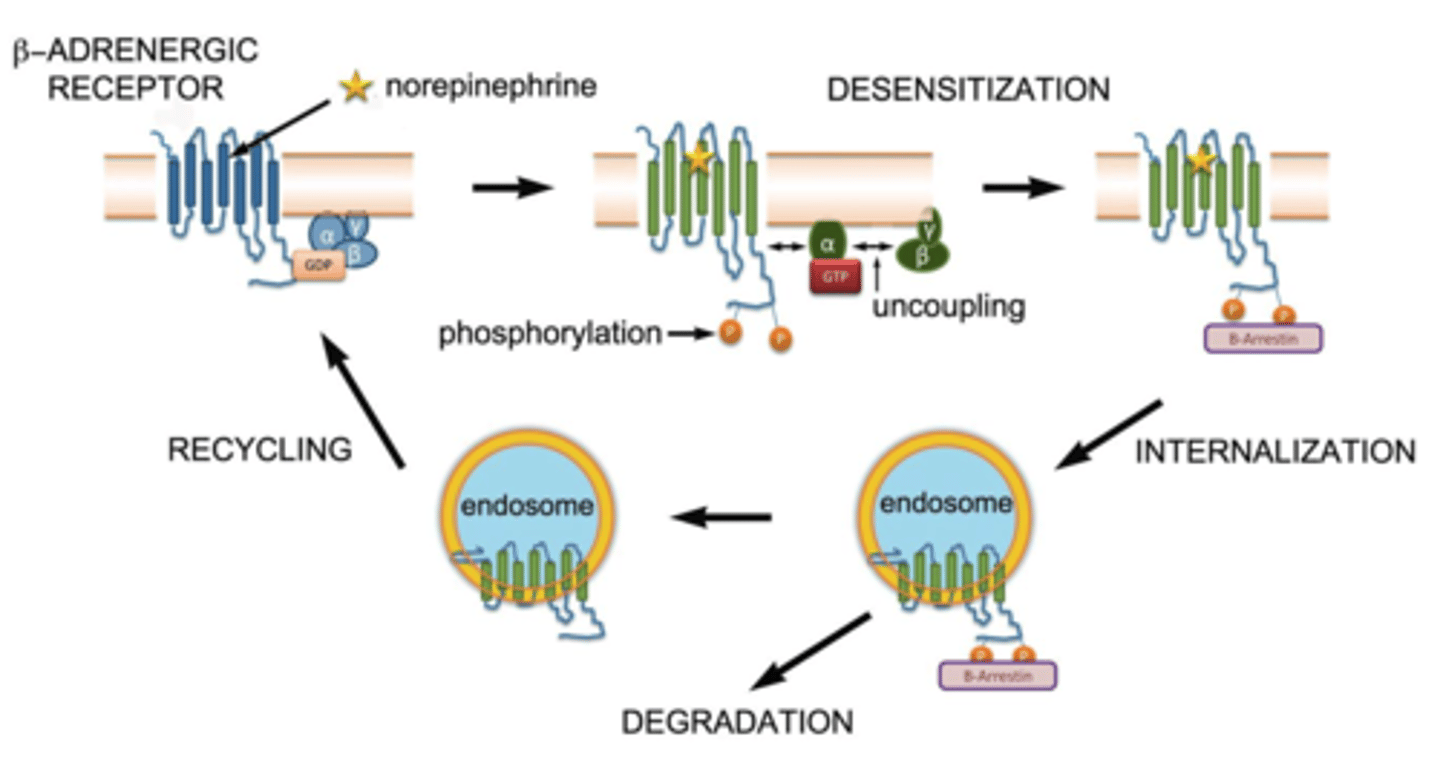
GPCR internalization/ desensitization
internalization: receptor pulled into cell → recycled or degraded
desensitization: receptor less responsive → GRK phosphorylates → arrestin blocks signaling
prevents receptor overstimulation
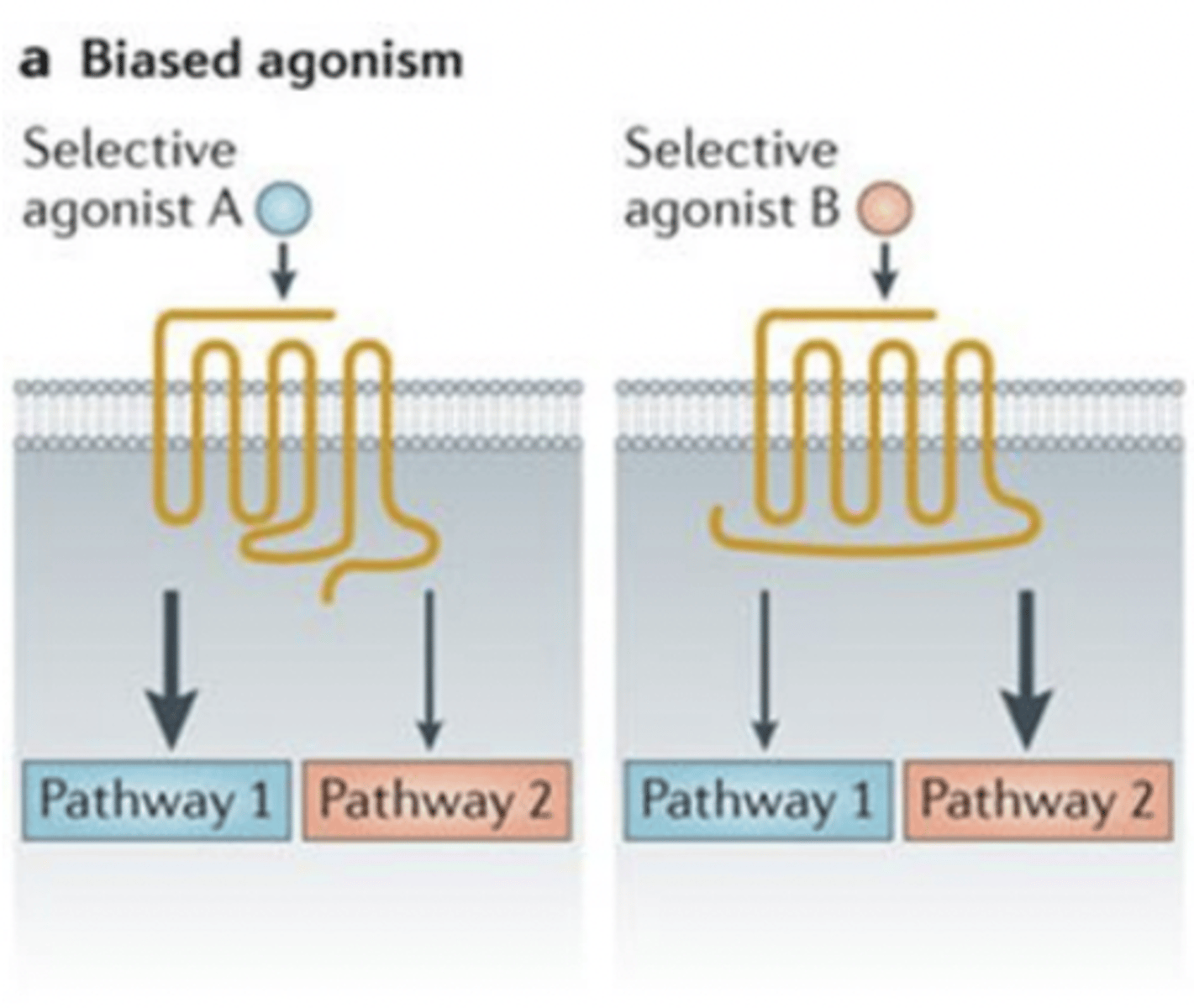
biased agonism
ligand preferentially activates one signaling pathway over another (e.g., G protein vs. arrestin).
- can fine-tune drug effects → more therapeutic, fewer side effects.
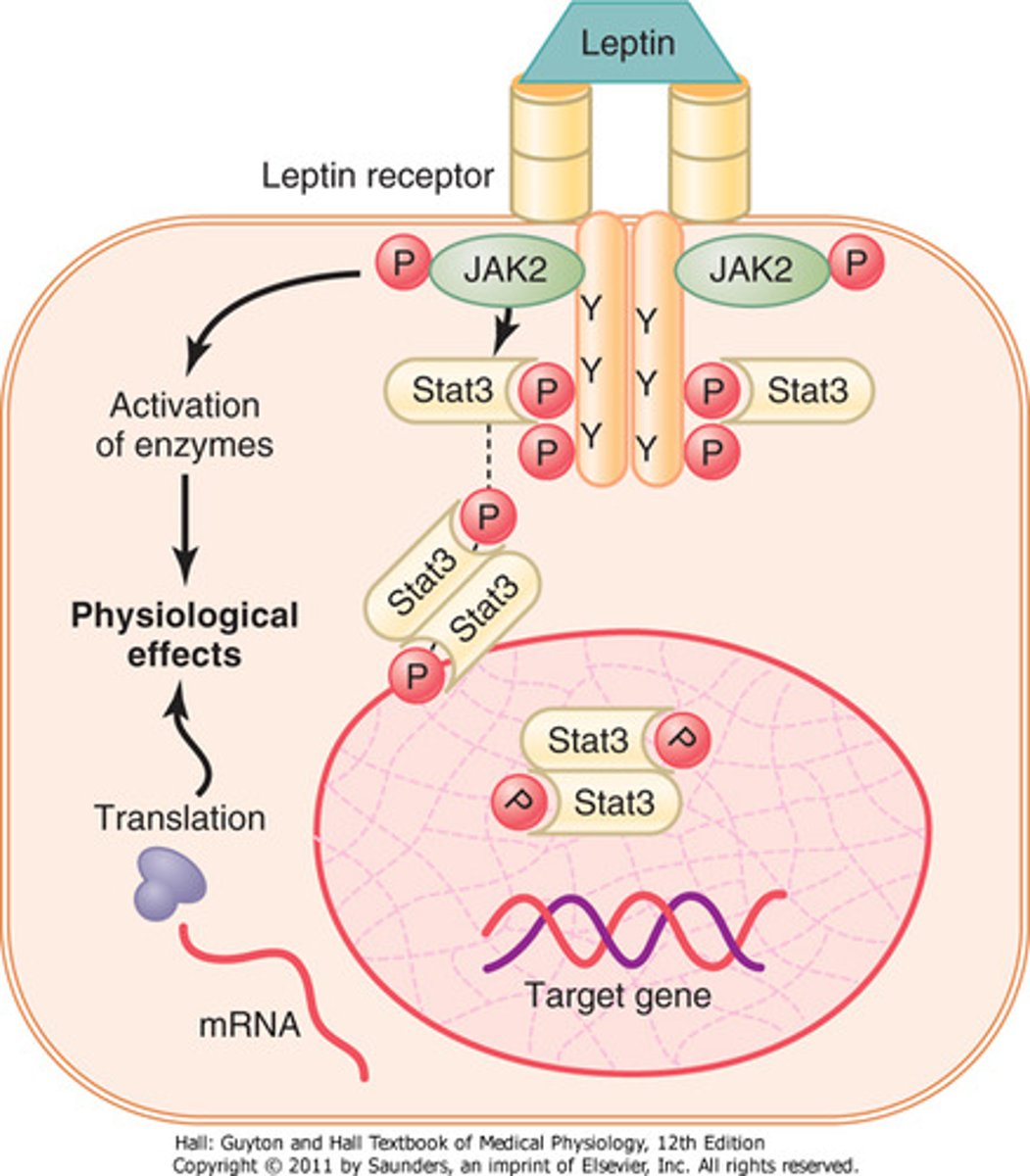
enzyme-linked hormone receptors
some receptors have intrinsic enzyme activity and don't need 2nd messengers to work
- enzymatic activity may come directly from the receptor interaction (on cytosolic side) OR from an enzyme closely associated with the receptor
-ex: leptin receptor, insulin receptor
tyrosine derivatives: catecholamines
hormone class
- synthesized: from tyrosine (amino acid) in the adrenal medulla
- stored: in secretory granules
- solubility: water soluble, ~50% circulate free, ~50% loosely conjugated (albumin)
- t1/2: 1-3 mins
- receptor: GPCR
- ex: dopamine, norepinephrine, epinephrine
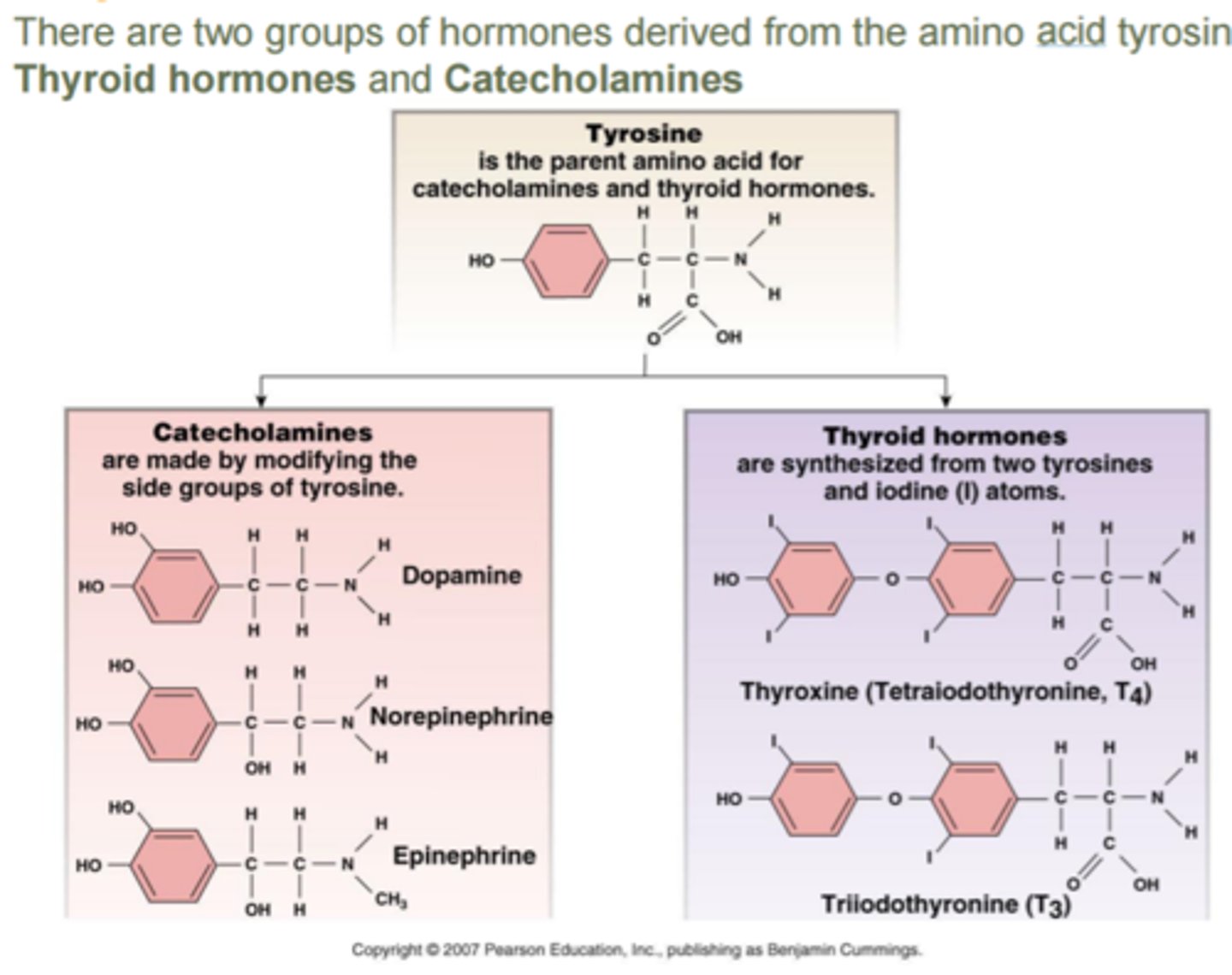
tyrosine derivatives: thyroid hormones
hormone class
- synthesized: from tyrosine (amino acid) in the thyroid gland
- stored: in thyroid gland (bound to thyroglobulin)
- released: by diffusion in response to thyroid-stimulating hormone (TSH)
- solubility: bound to thyroxine
- t1/2: T3=2.5 days T4=6-7 days
- receptor: intracellular (cytosolic or nuclear)
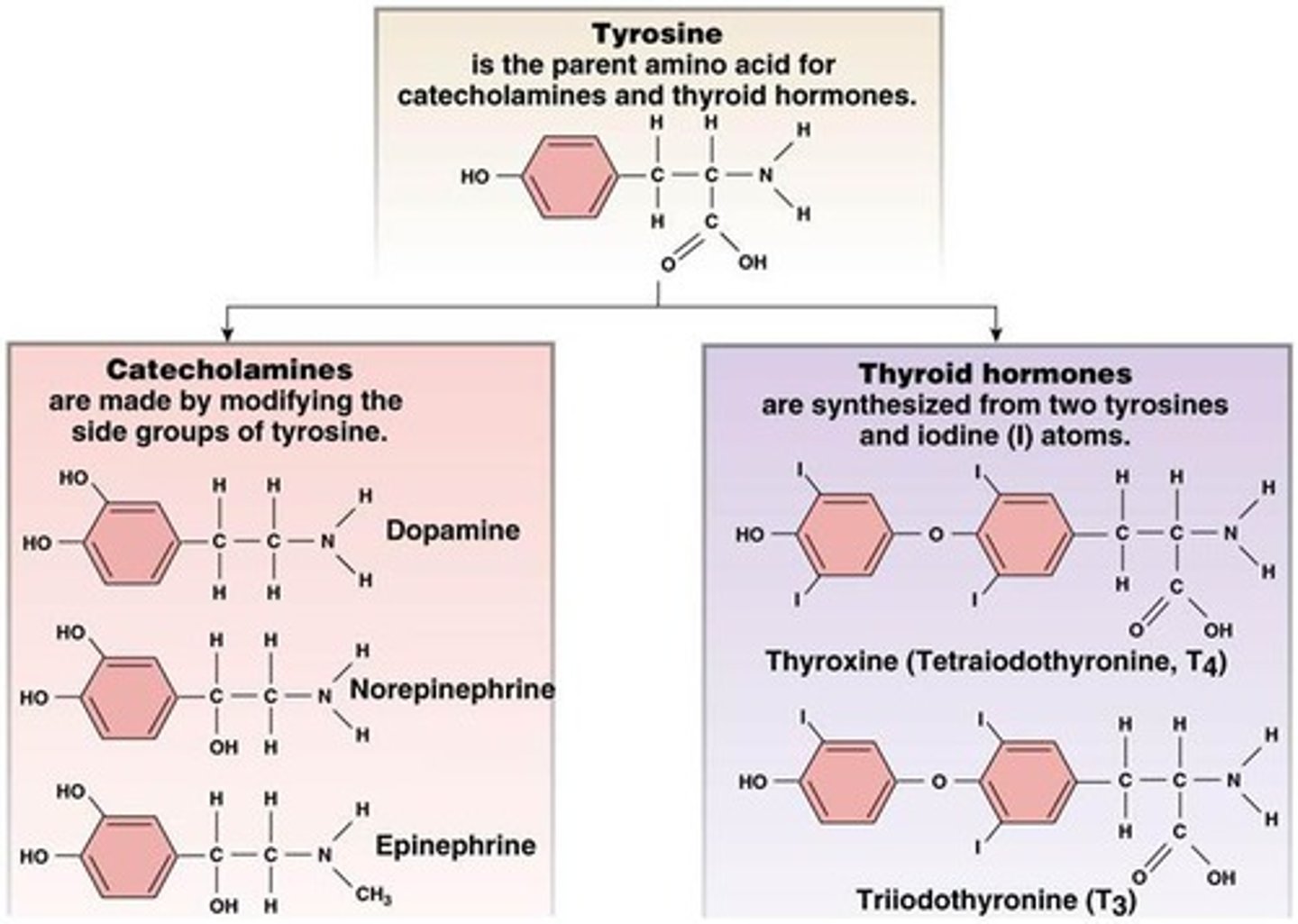
3 classes of hormones review
steroids:
- no storage
- diffuses across cell membrane
- enter interstitial fluid -> blood stream
- 90% bound to albumin/ binding globulins in plasma
peptides:
- generally stored as prohormones
- secretory vesicles fuse w cell membrane -> contents extruded into interstitial fluid or blood stream by exocytosis
catecholamines:
- stored in secretory granules
- rapid burst in response to stimuli
- soluble in plasma -> rapidly metabolized
hormone binding review
- affects blood levels
- bound hormone = inactive and protected from degradation (serves as hormone reservoir)
- depends on amount of binding protein available
- can also be taken up into tissues or bound to receptors
clearance review
hormones are cleared by:
- metabolic destruction by enzymes in blood/ tissues
- binding within tissues
- excretion by liver into bile
- excretion by the kidneys into urine
half-lives:
- steroids - hours
- catecholamines - seconds
- peptides - minutes
(steroids > thyroid hormones > peptides > catecholamines)
endocrine hypothalamus
- functions as a command center integrating information from the body and its environment
- most daily functions are influenced by hypothalamic signals + responses in the pituitary
- regulation is dynamic and adapts to changes
hypothalamus signals
signals to the pituitary to secrete hormones
- if the hormone ends in -rh, it comes from the hypothalamus (TRH, Gnrh, gh-rh)
pituitary structure
anterior and posterior lobe
- sit below the hypothalamus
- 2 hormones (oxytocin and vasopressin) are produced in the hypothalamus and stored in the posterior pituitary
- 6 hormones are secreted by the anterior pituitary
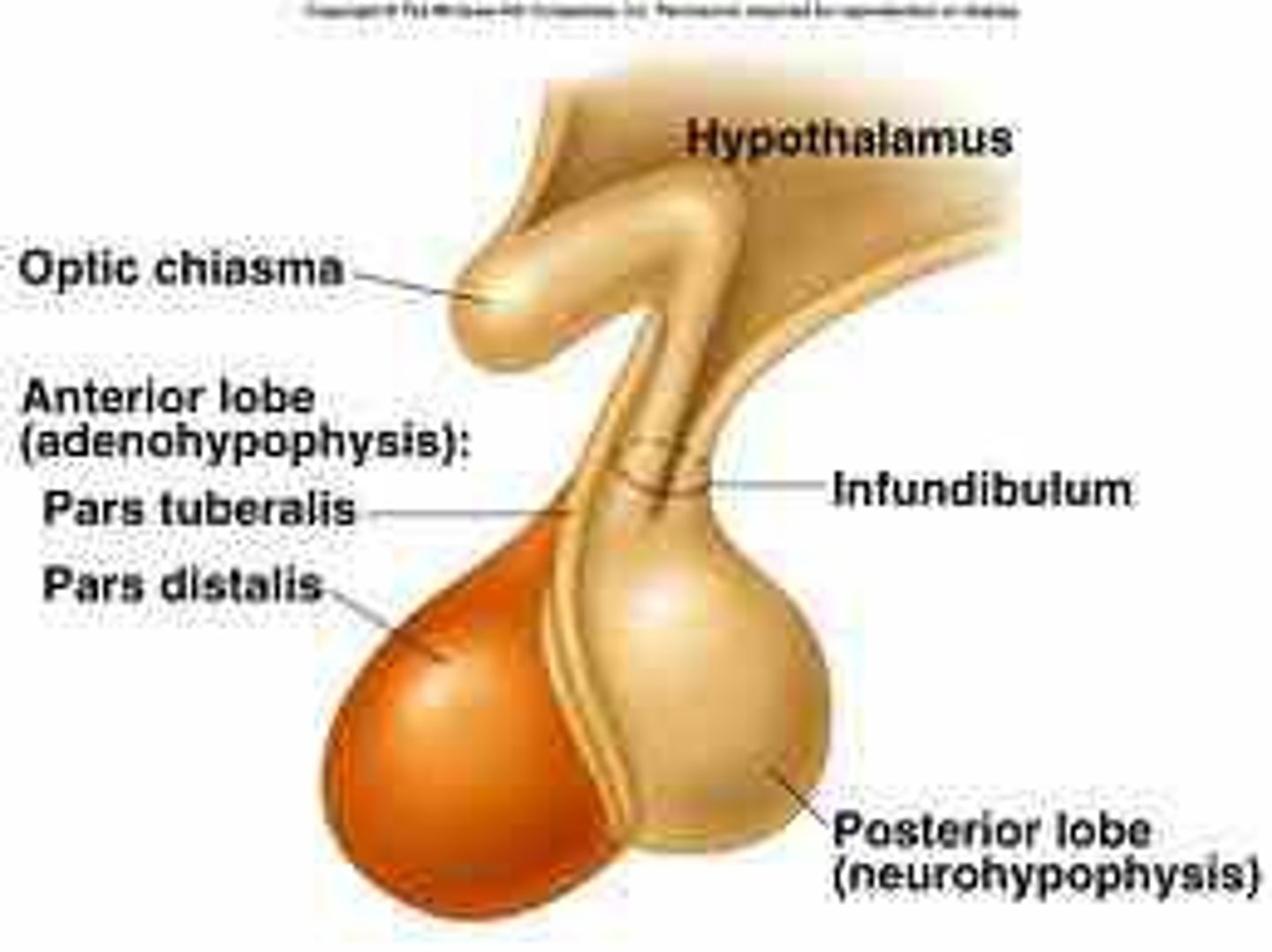
releasing hormones (RH)
- synthesized via Parvicellular neurons and travel via a portal system to the anterior pituitary -> stimulate production of pituitary trophic hormones
- posterior pituitary hormones are produced by magnocellular neurons in the hypothalamus -> travel to the posterior pituitary to be stored in nerve terminal vesicles
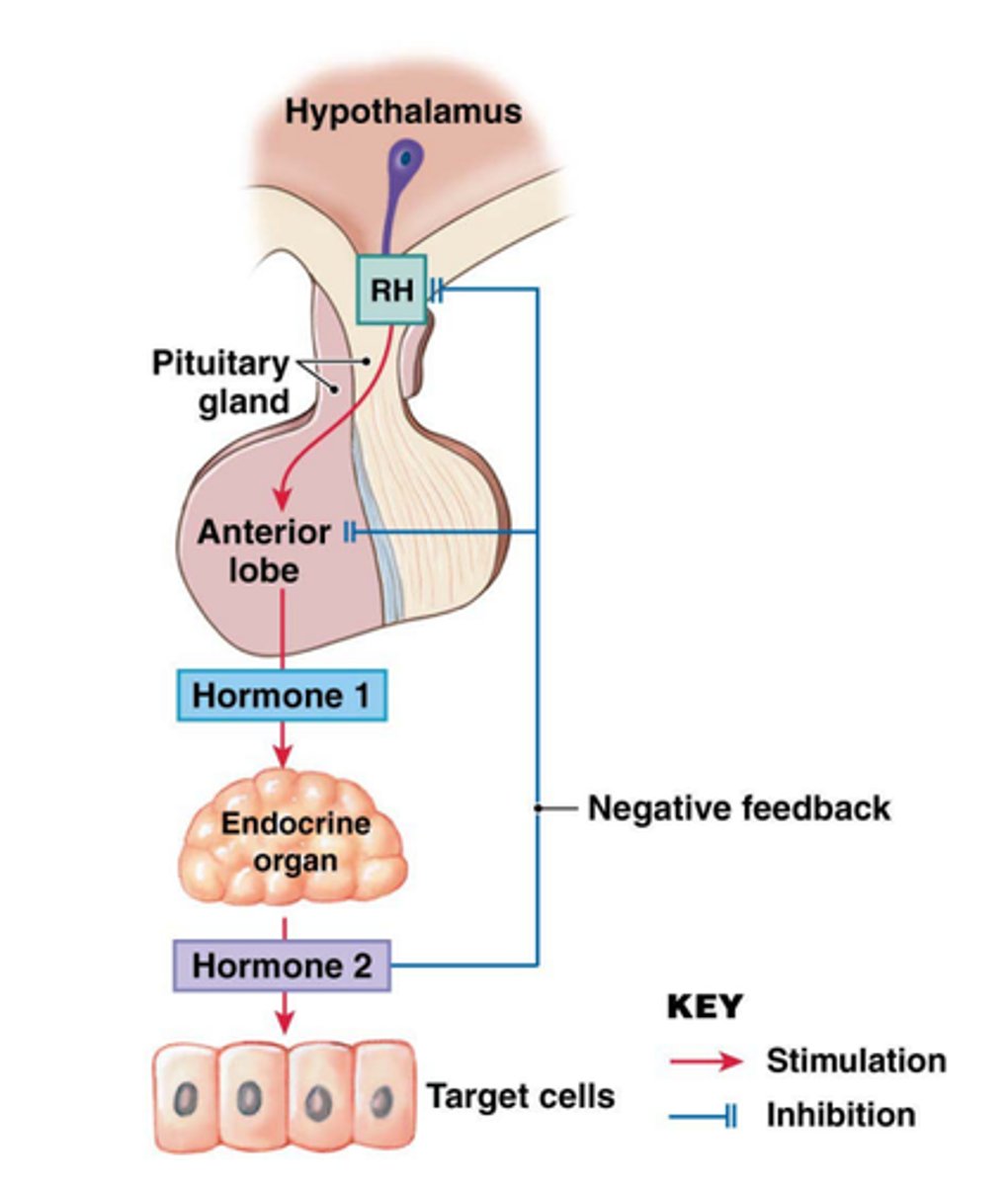
the pituitary gland has a portal vascular system similar to...
the liver
pulsatile vs circadian secretion
pulsatile: intermittent bursts over short periods of time
- ex: LH release from anterior pituitary
circadian: follows a 24 hour cycle
- ex: cortisol peaks in the morning and decreases through the day
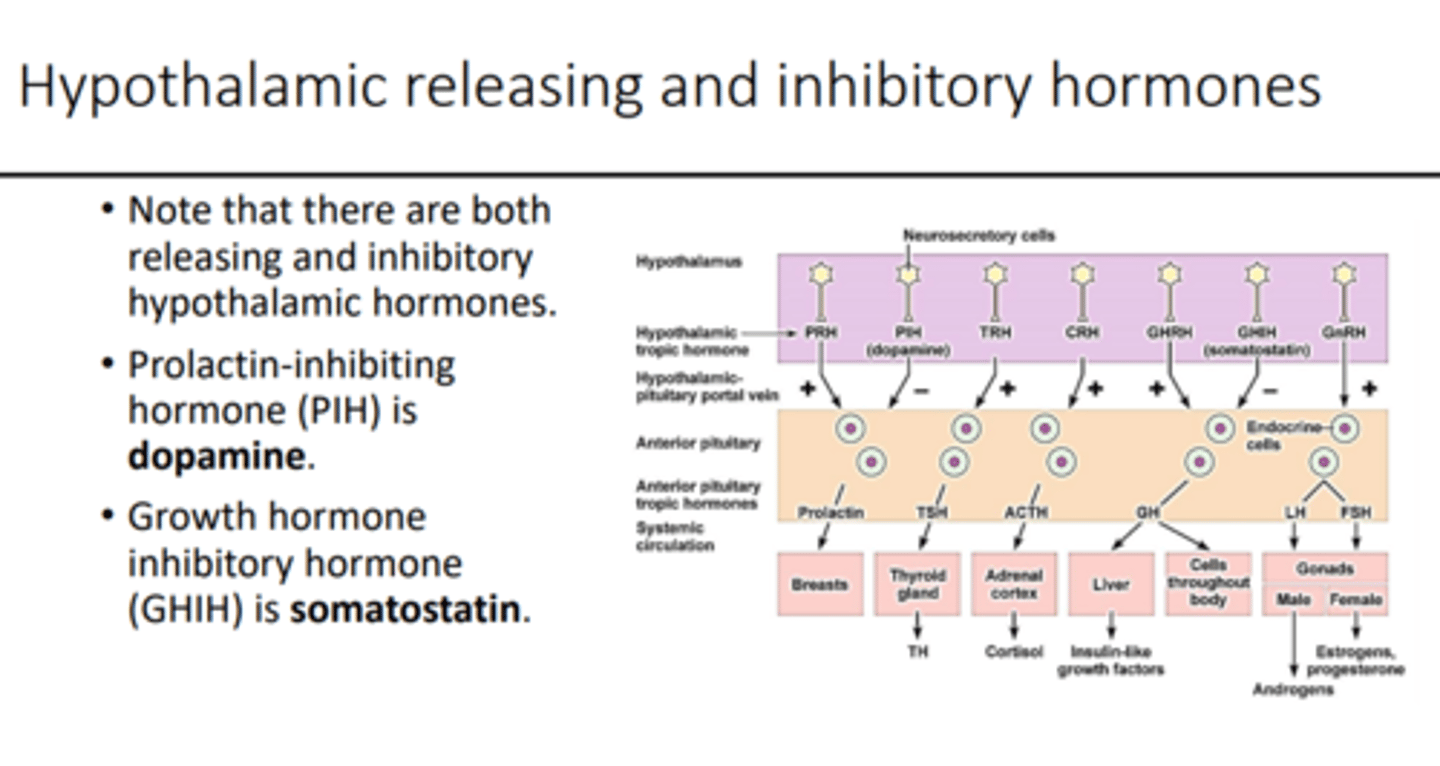
hypothalamic releasing stimulatory hormones
1. thyroid-releasing hormone (TRH)
- peptide of 3 AA
- stimulates secretion of TSH
2. gonadotropin-releasing hormone (GnRH)
- single chain of 10 AA
- stimulates secretion of FSH and LH
3. corticotropin-releasing hormone (CRH)
- single chain of 41 AA
- stimulates secretion of ACTH
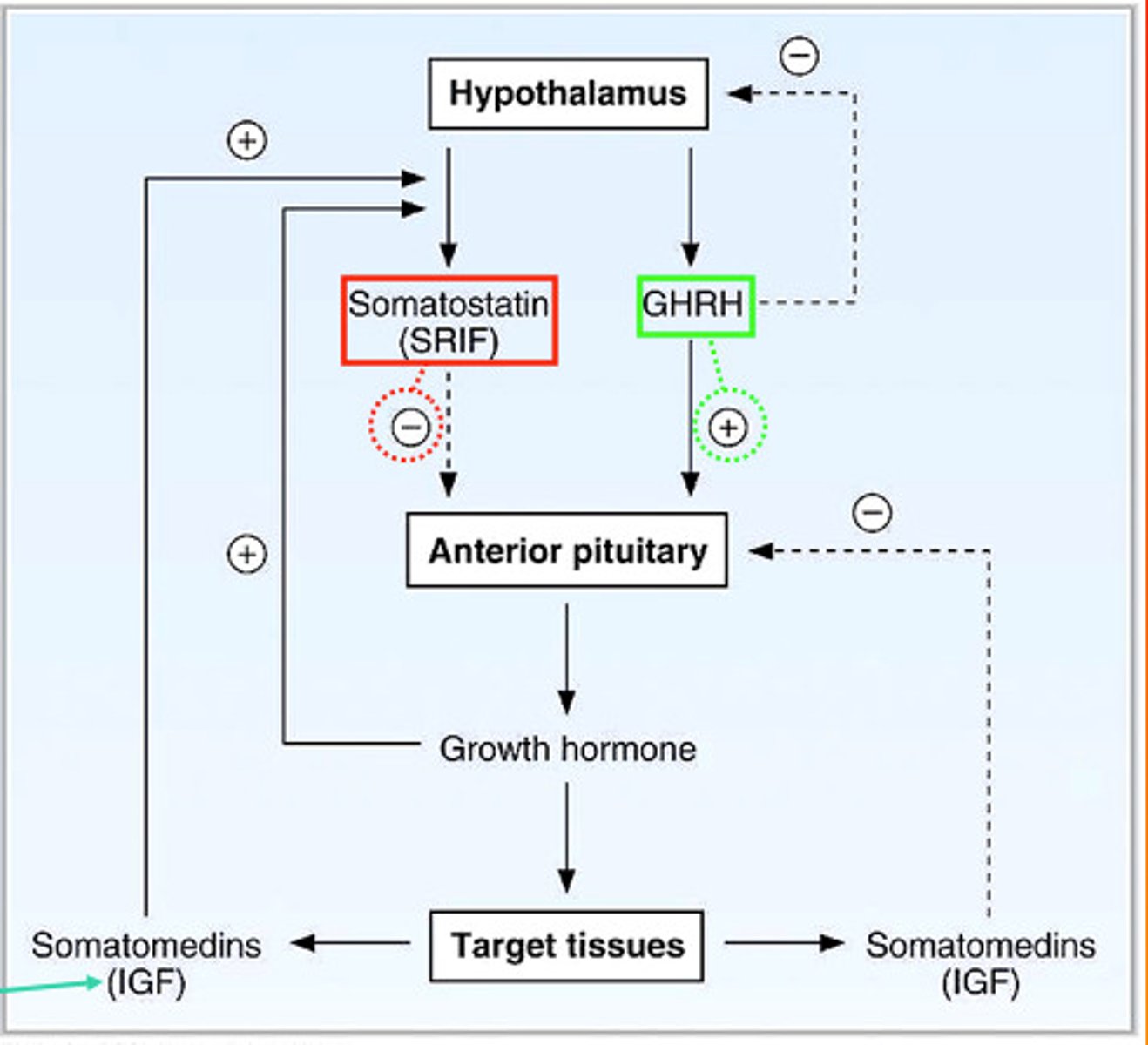
4. growth hormone-releasing hormone (growth hormone RH)
- single chain of 44 AA
- stimulates secretion of growth hormone
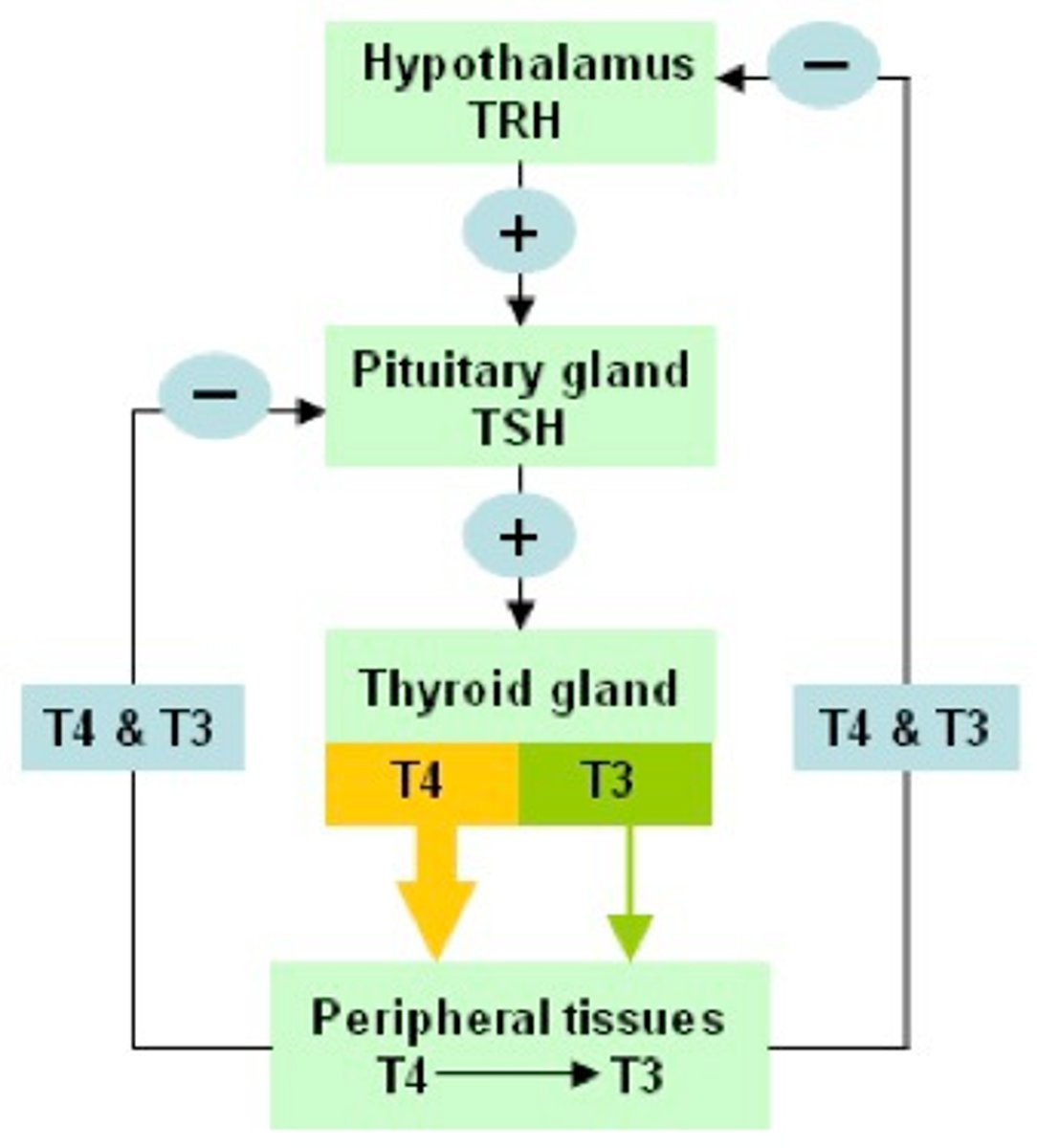
hypothalamic releasing inhibitory hormones
1. growth hormone inhibitory hormone (somatostatin)
- single chain of 14 AA
- inhibits secretion of growth hormone
2. prolactin-inhibiting hormone (PIH)
- dopamine
- inhibits secretion of prolactin
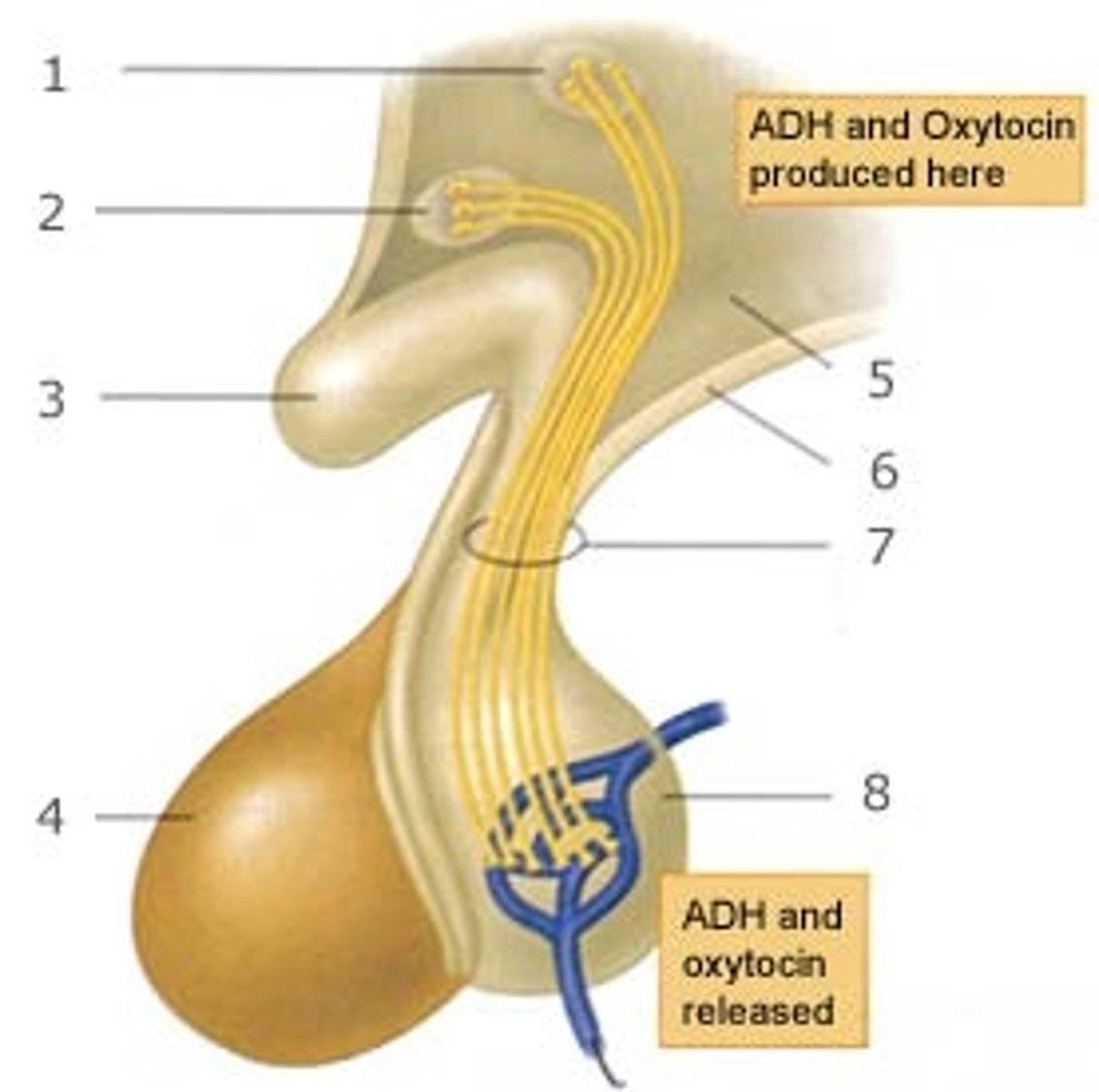
posterior pituitary hormones
1. vasopressin (antidiuretic hormone, ADH)
- formed in supraoptic nucleus of hypothalamus
- controls blood pressure, rate of water excretion
- stimulated by: low BP, salty/low water, RAS activation
2. oxytocin
- formed in paraventricular nucleus of hypothalamus
- positive feedback effect during child labor, milk expression
- have similar structures
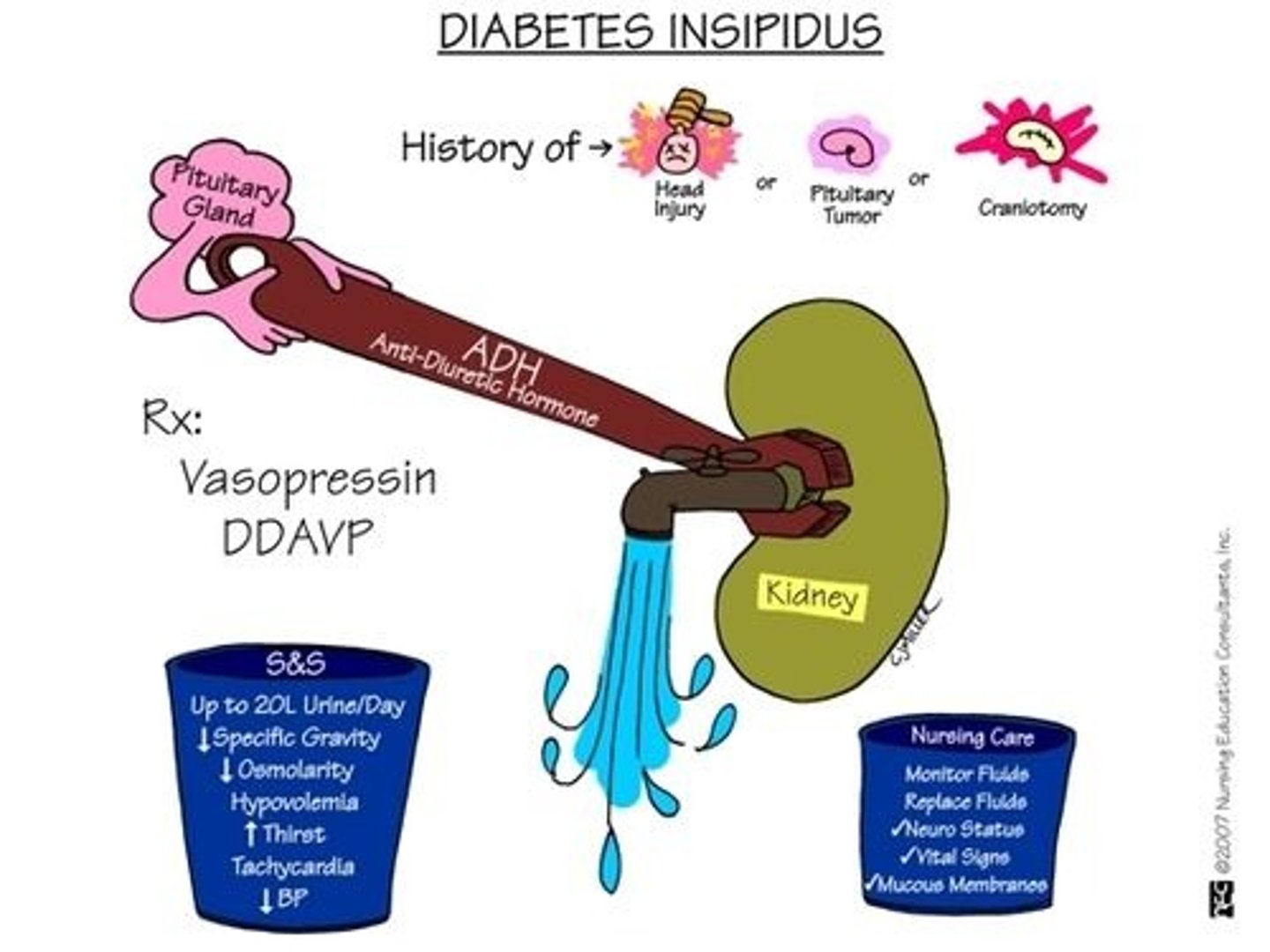
diabetes insipidus
deficiency of vasopressin (antidiuretic hormone, ADH)
- may be neurogenic or nephrogenic
- treatment: desmopressin (synthetic ADH)
growth hormone
AKA somatotropin
hormone secreted by anterior pituitary gland that stimulates growth
- a protein composed of 191 AAs
- similar structure to prolactin
- t1/2: 20 minutes
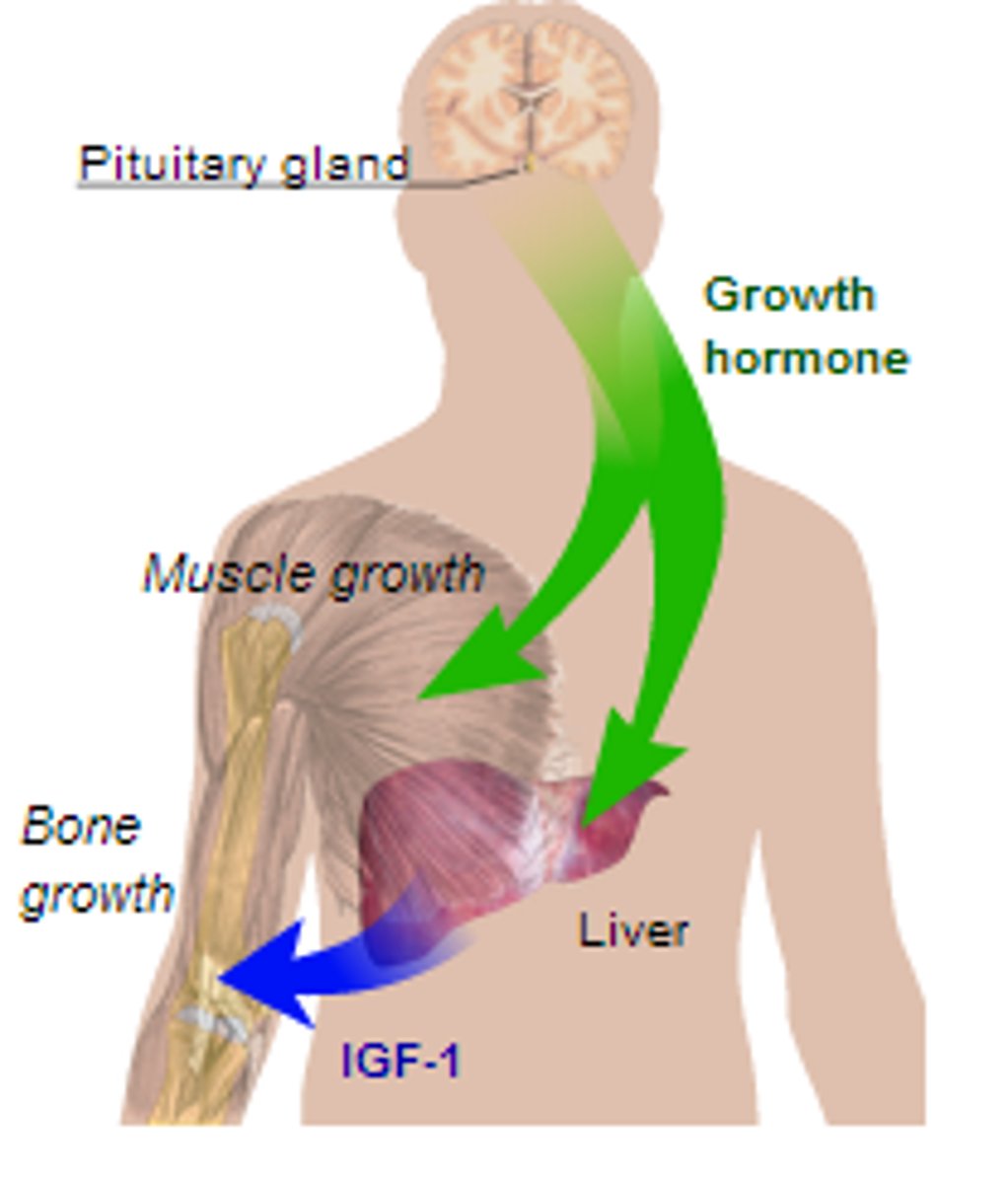
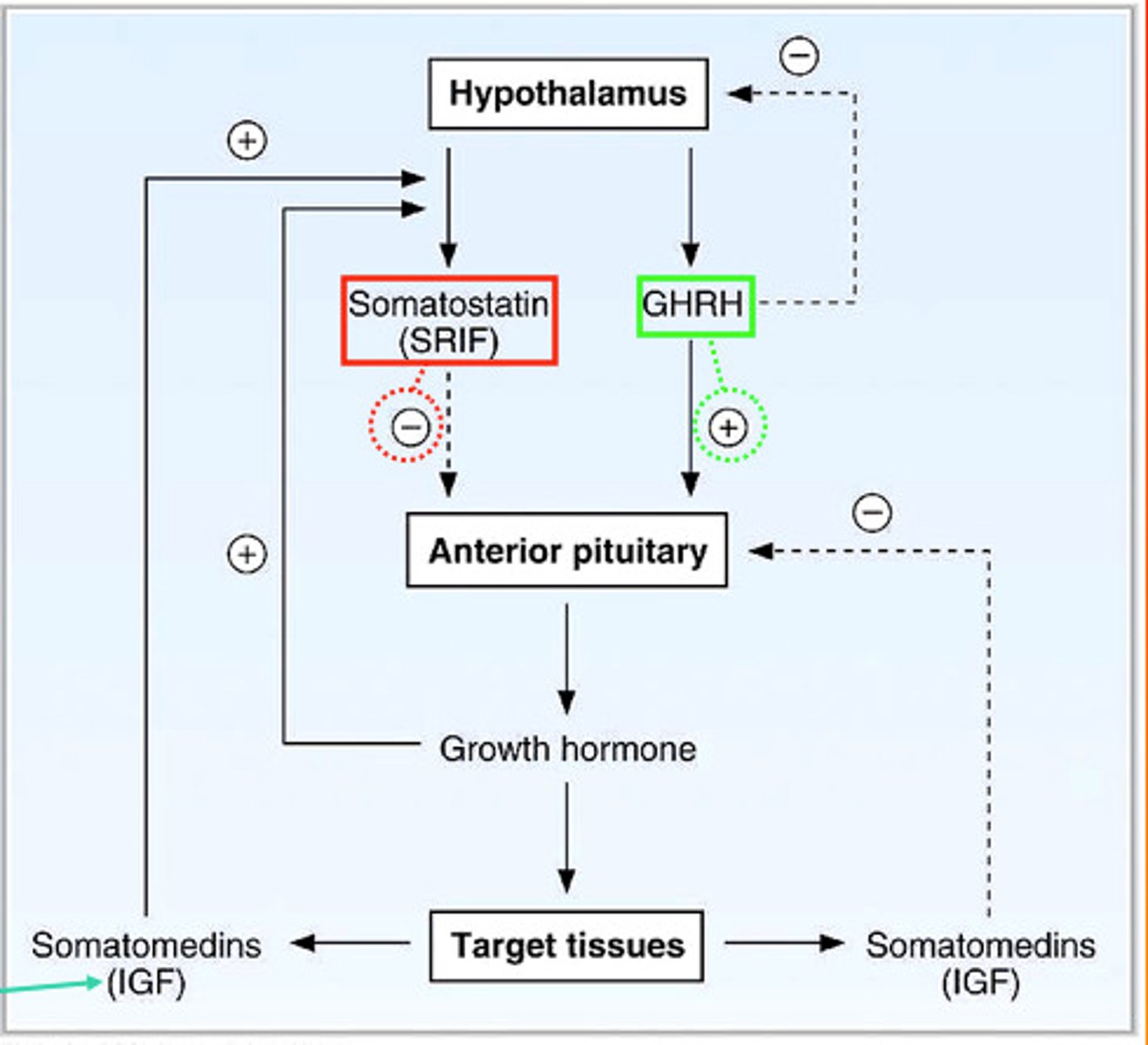
growth hormone regulation
the hypothalamus produces:
growth hormone-releasing hormone (GH-RH)
and
growth hormone inhibitory hormone (somatostatin)
- acts directly on body tissues (liver, adipose, bone, muscle)
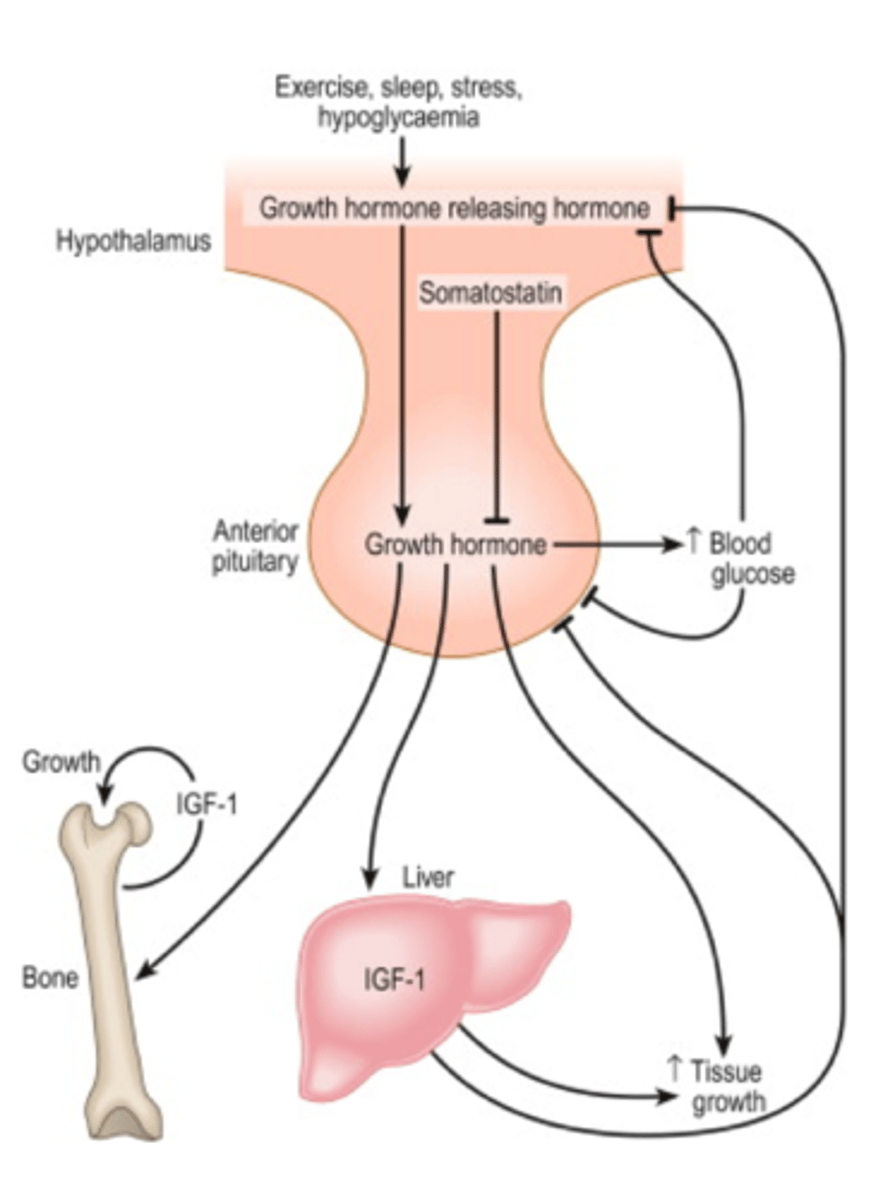
somatotropin vs somatostatin
somatotropin: growth hormone (GH)
- a protein hormone produced by the anterior pituitary gland
somatostatin: growth hormone-releasing inhibiting factor (GH-RF)
- a peptide hormone produced by the hypothalamus
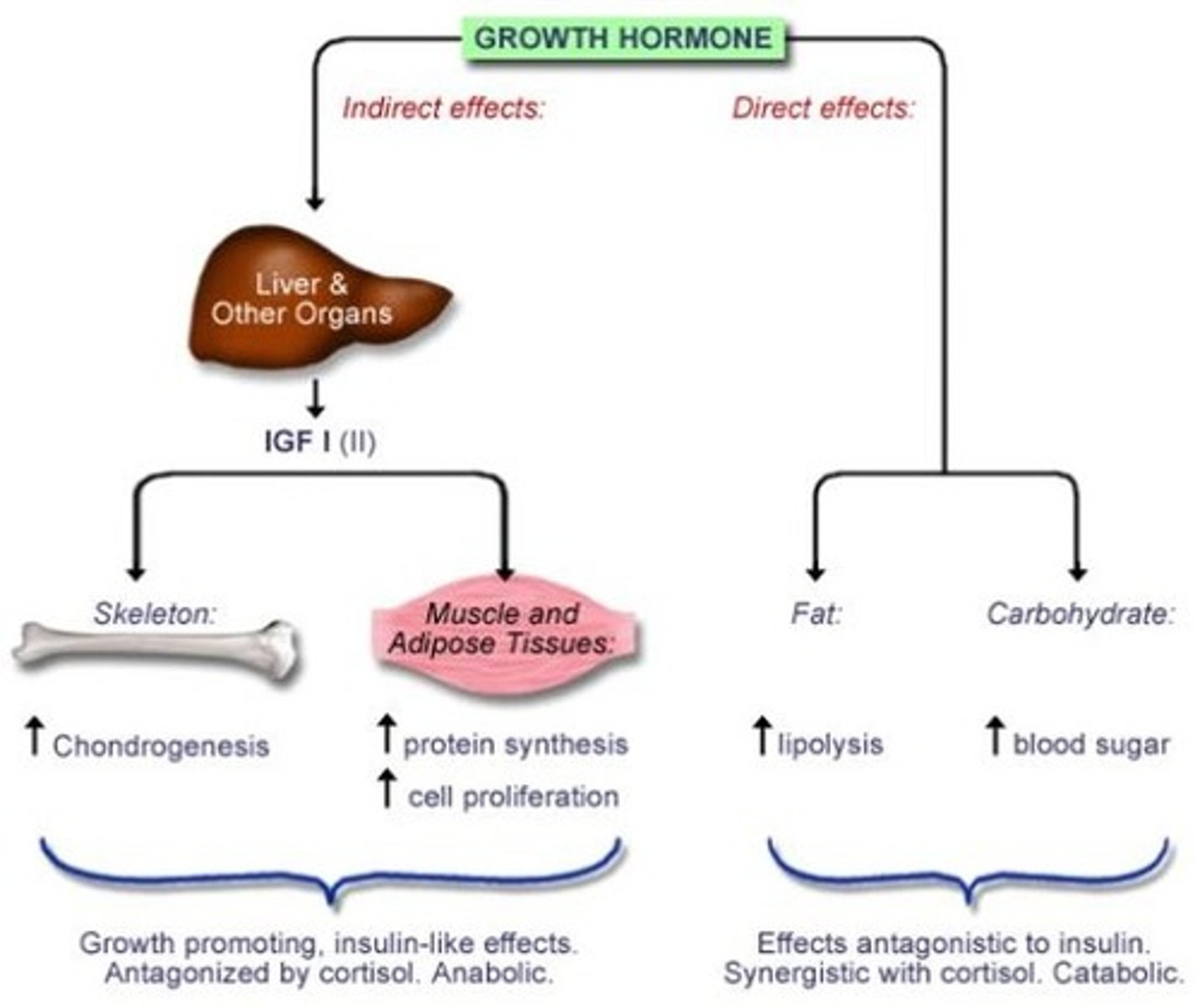
effects of growth hormone
direct effects on: adipose, liver, skeletal, bone
- bone elongation promoted at epiphyseal plates
indirect effects via IGF-1: ↑ glucose uptake, ↑ AA synthesis, ↑ protein synthesis
life-time pattern of growth hormones
greatest at puberty
declines in advanced age
3 disorders of growth hormone (GH) release
1. gigantism - excess GH before puberty
- primarily due to anterior pituitary tumor
2. dwarfism - GH deficiency before puberty
- panhypopituitarism - decrease secretion of ALL pituitary hormones
3. acromegaly - excess GH after puberty
- bones thicken, soft tissues grow

obesity
having an excess amount of body fat
- due to: appetite dysregulation, abnormal energy balance, endocrine dysfunction
- associated symptoms: joint pain, altered metabolism, sleep apnea
- leads to: T2DM, CV, cancer, osteoporosis, PCOS, infertility, NAFLD/MAFLD, dyslipidemia
how to calculate BMI
weight (kg) / height (m^2)
lb → kg = lb/2.2
ft → in
in → cm = in x 2.54
cm → m = cm/100
m^2

BMI classification of obesity
underweight <18.5
normal 18.5 - 25
pre-obesity 25- 30
obesity ≥30
- obesity class I 30 - 35
- obesity class II 35 - 40
- obesity class III ≥40
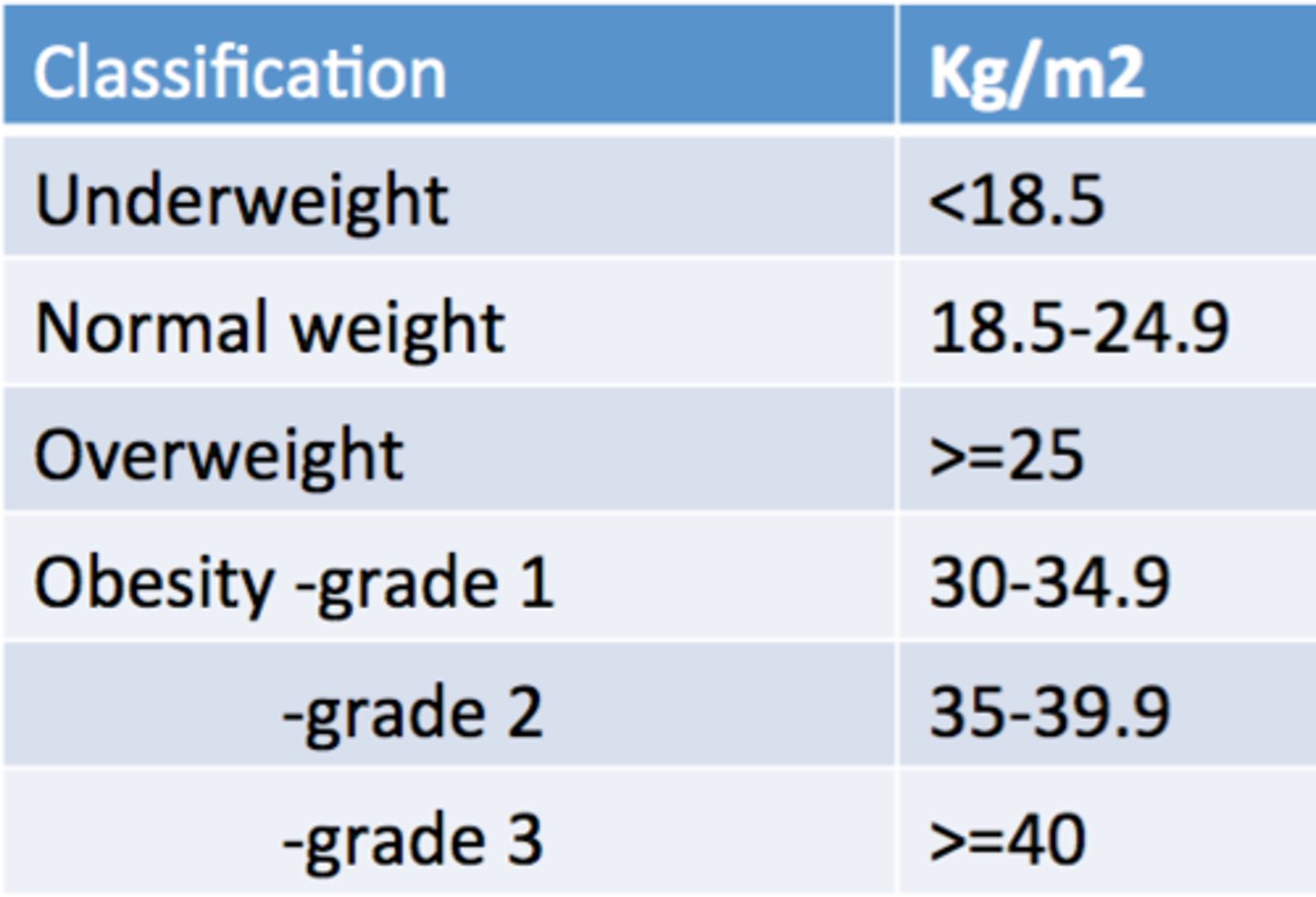
what BMI classifies someone as obese?
≥30
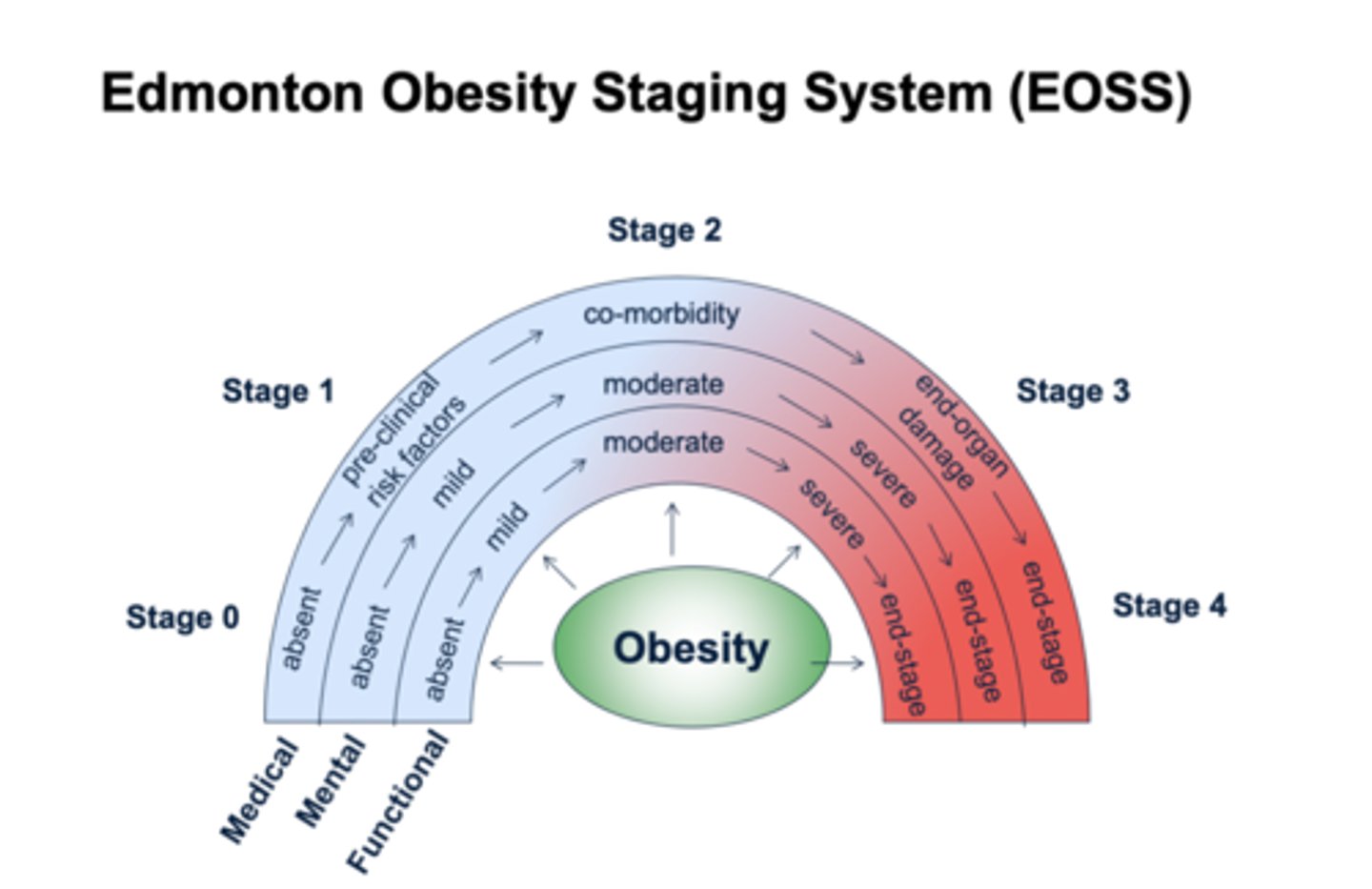
edmonton obesity staging system (EOSS)
classes obesity based in morbidity and functional limitations
- stage 0-4 with 4 being end stage for medical, mental, and functional complications
- used to predict risks and benefits for obesity management
why do some people gain weight easily and others don't?
- genetics
- behavioral factors
- hormonal factors
- sleep patterns
- eating habits
highest prevalence of adult obesity in what population?
non-hispanic black
is obesity a chronic disease?
yes, and requires long-term management + ongoing care and support
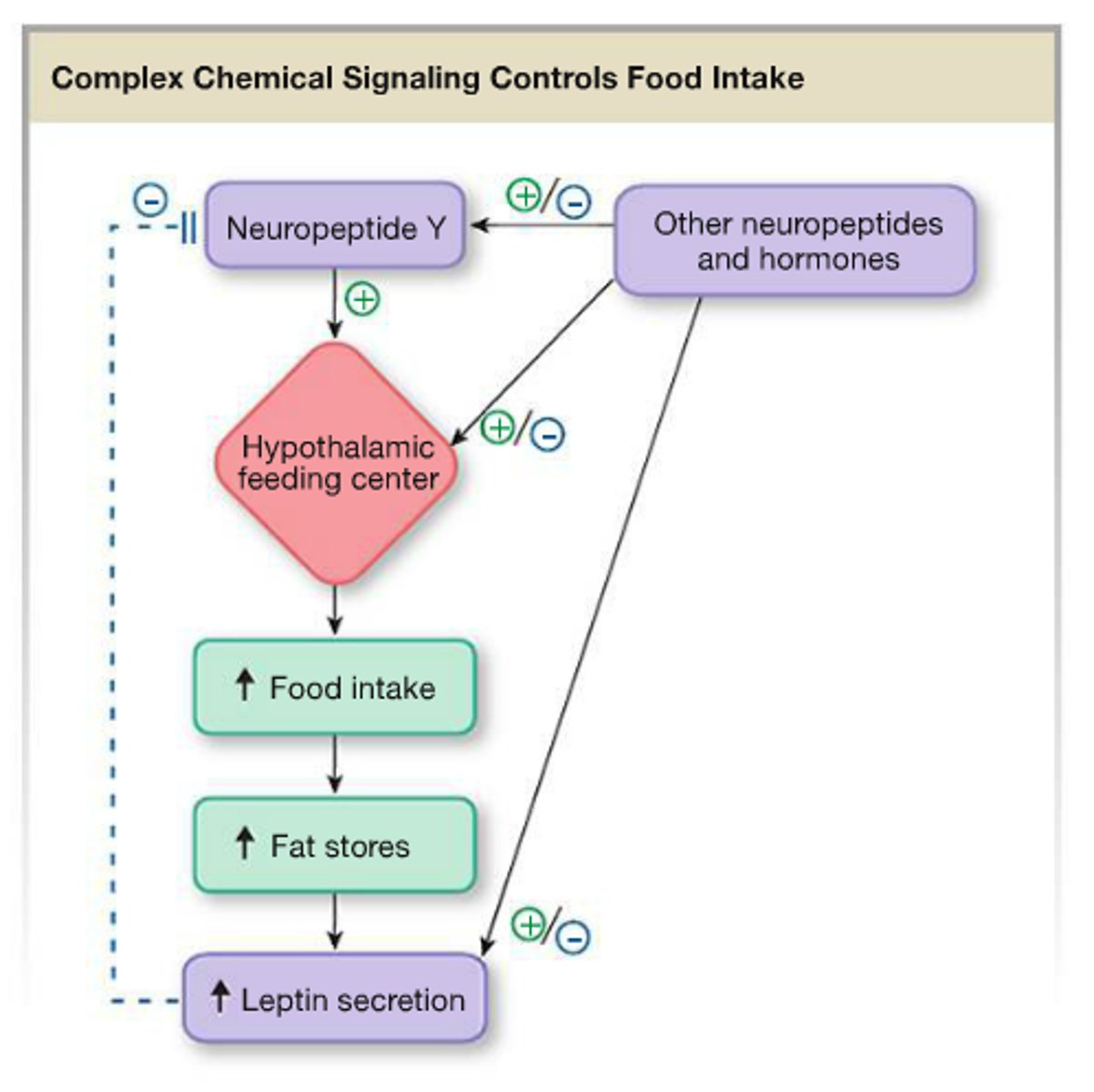
hormone appetite signaling
orexigenic (increases appetite)
- ghrelin
anorexigenic (decreases appetite)
- CCK
- GLP-1
- GIP
- amylin/ insulin/ glucagon
- PP
- leptin
- adiponectin
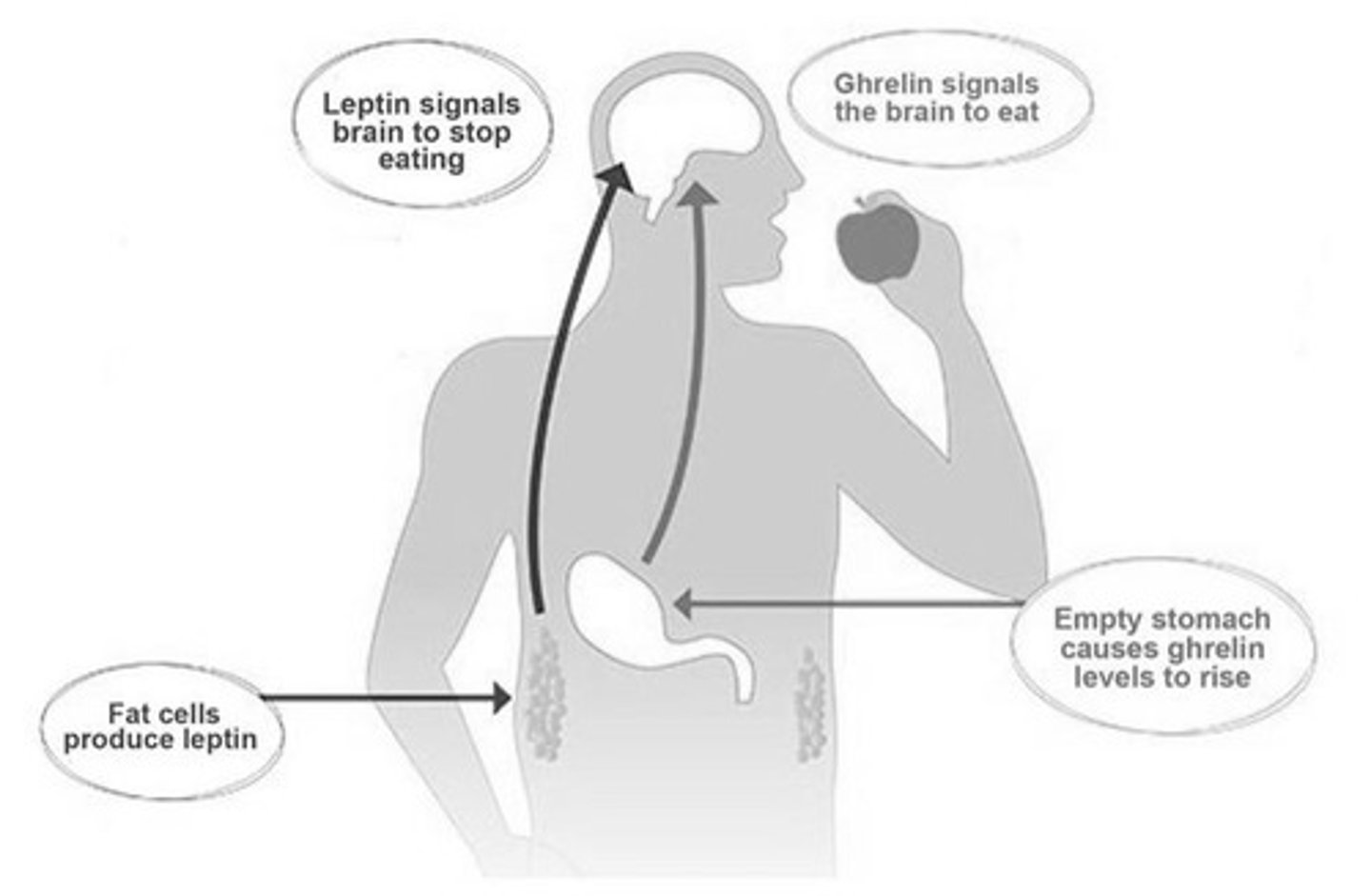
3 types of eating
1. homeostatic: eating for hunger
- GLP-1, GIP, amylin, PYY ↑ satiety; ghrelin ↑ appetite
2. hedonic: eating for pleasure
- cannbinoid, opioid, and dopamine receptors involved
3. executive function: deciding to eat
- higher order brain regions override homeostatic drive
- thoughts ⇌ behavior ⇌ feelings
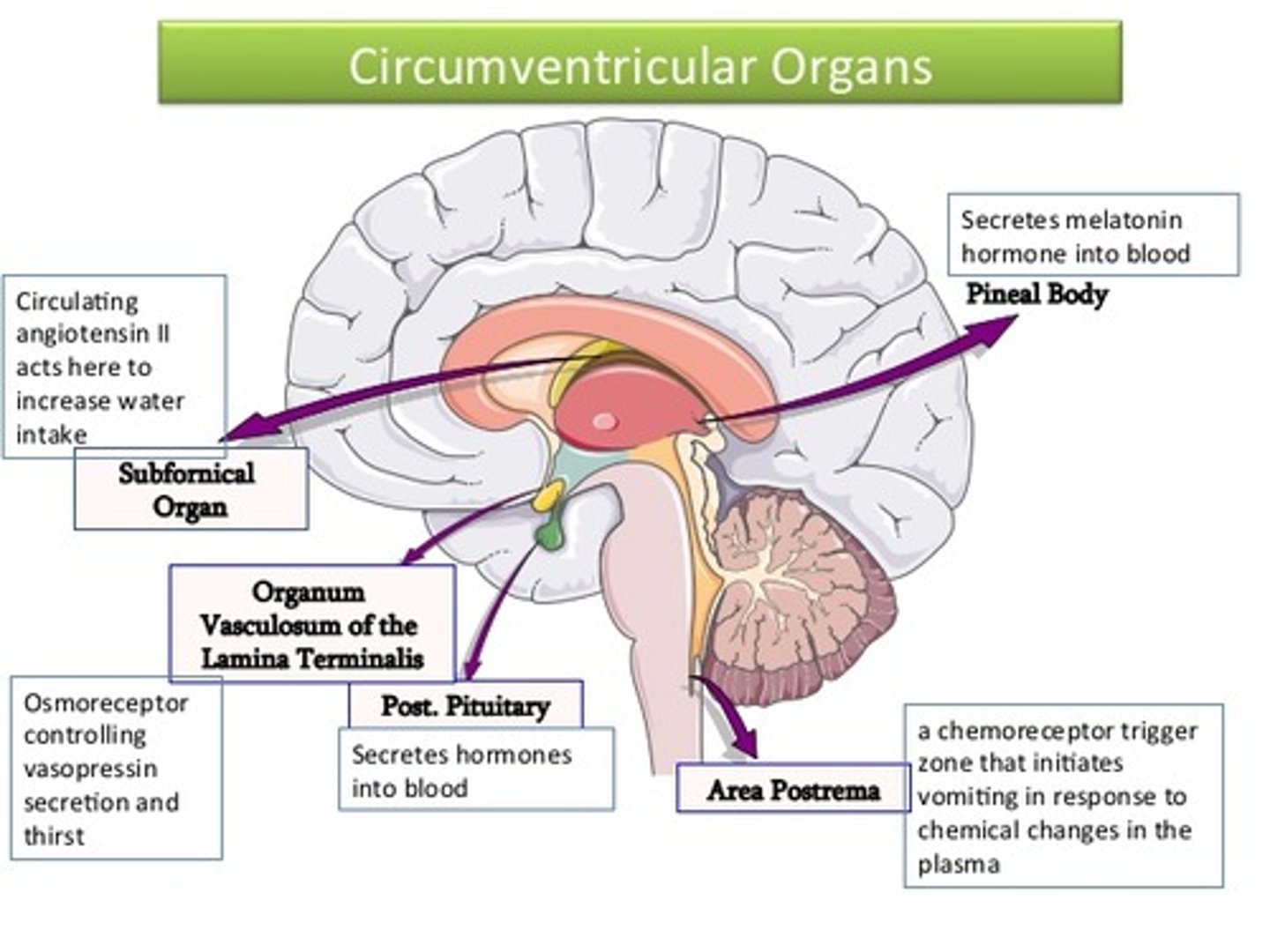
hunger and satiation signals in the brain
1. circumventricular organs
- a "window" in the brain with no BBB = can sense nutrients and hormones in circulation
↓
2. ventricle
- circulating CSF contains metabolic signals
↓
3. nucleus tractus solitarius
- vagus afferent nerve terminates and sends meal information straight from the gut
+
4. arcuate nucleus
- drug target neuron populations:
> POMC/CART - satiation
> NPY/AgRP - hunger
hypothalamic appetite signaling
arcuate nucleus (ARC): key appetite regulation center
NPY/AgRP neurons: ↑ appetite (orexigenic) → inhibit satiety pathway (POMC/CART).
POMC/CART neurons: ↓ appetite (anorexigenic) → activate MC4R in PVN to suppress feeding.
- balance between these pathways regulates food intake.
dorsal vagal complex (hindbrain) appetite signaling
- composed of NTS, area postrema, dorsal motor nucleus of vagus
- integrates gut-brain signals (CCK, GLP-1, vagal input)
- communicates with hypothalamus (ARC, PVN)
- helps regulate meal size & satiety
NTS neuronal populations appetite regulation
- central GLP-1 neurons: Inhibit food intake, signal satiety
- catecholaminergic (NE) neurons: Relay visceral sensory input, influence feeding
- other peptides: CCK, POMC, etc. contribute to satiety signal
t- he NTS integrates these → sends info to hypothalamus & dorsal vagal complex
obesity associated perturbations in gut-brain axis
brain:
- blunted neuronal activation
- inflammation, gliosis, and altered neuropeptide expression
- reduces sensing of vagal signals
gut:
- altered gut hormone release
- intestinal hyperpermeability
- microbial dysbiosis
- a resistance to satiation hormones, nutrients, and vagal afferent input in obesity → cannot sense body's nutrient needs
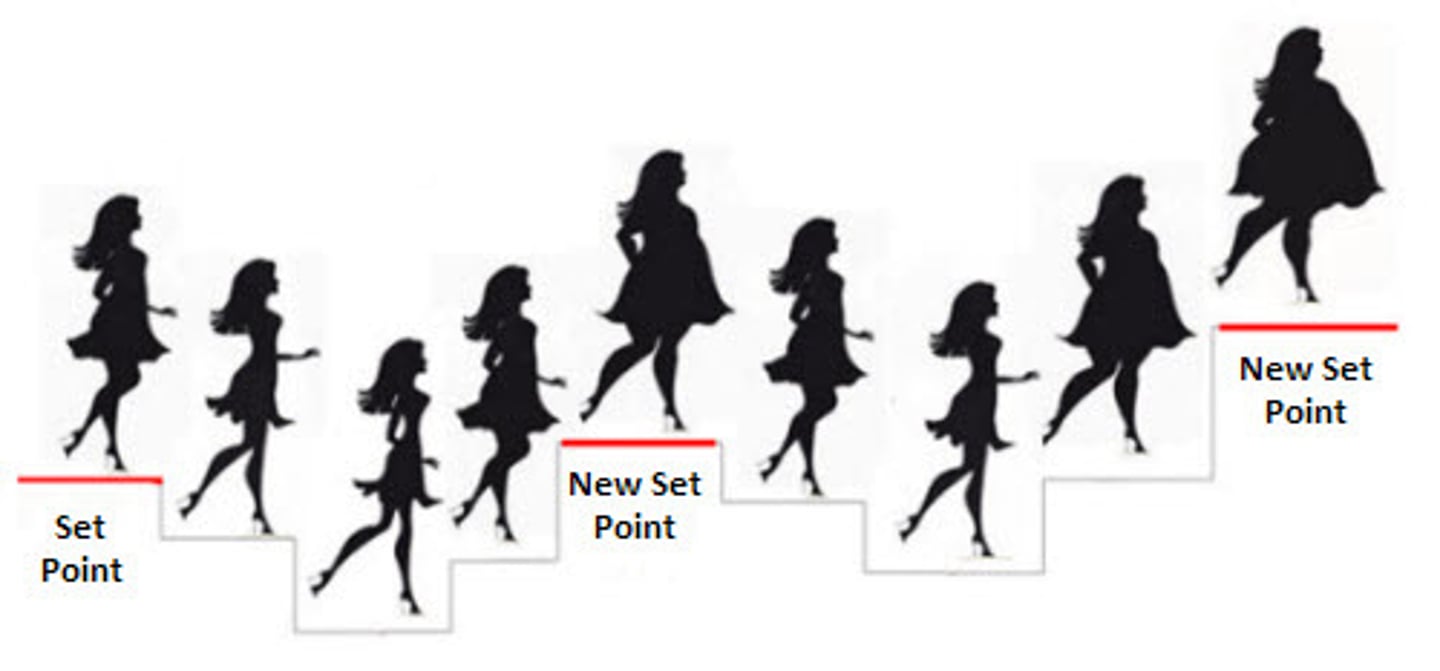
set point
the point at which an individual's "weight thermostat" is supposedly set
- when the body falls below this weight, an increase in hunger and a lowered metabolic rate may act to restore the lost weight
- takes months-years to challenge this
resting metabolic rate (RMR)
how much energy you burn at rest
- will decrease with weight loss as compensation to remain at set point weight
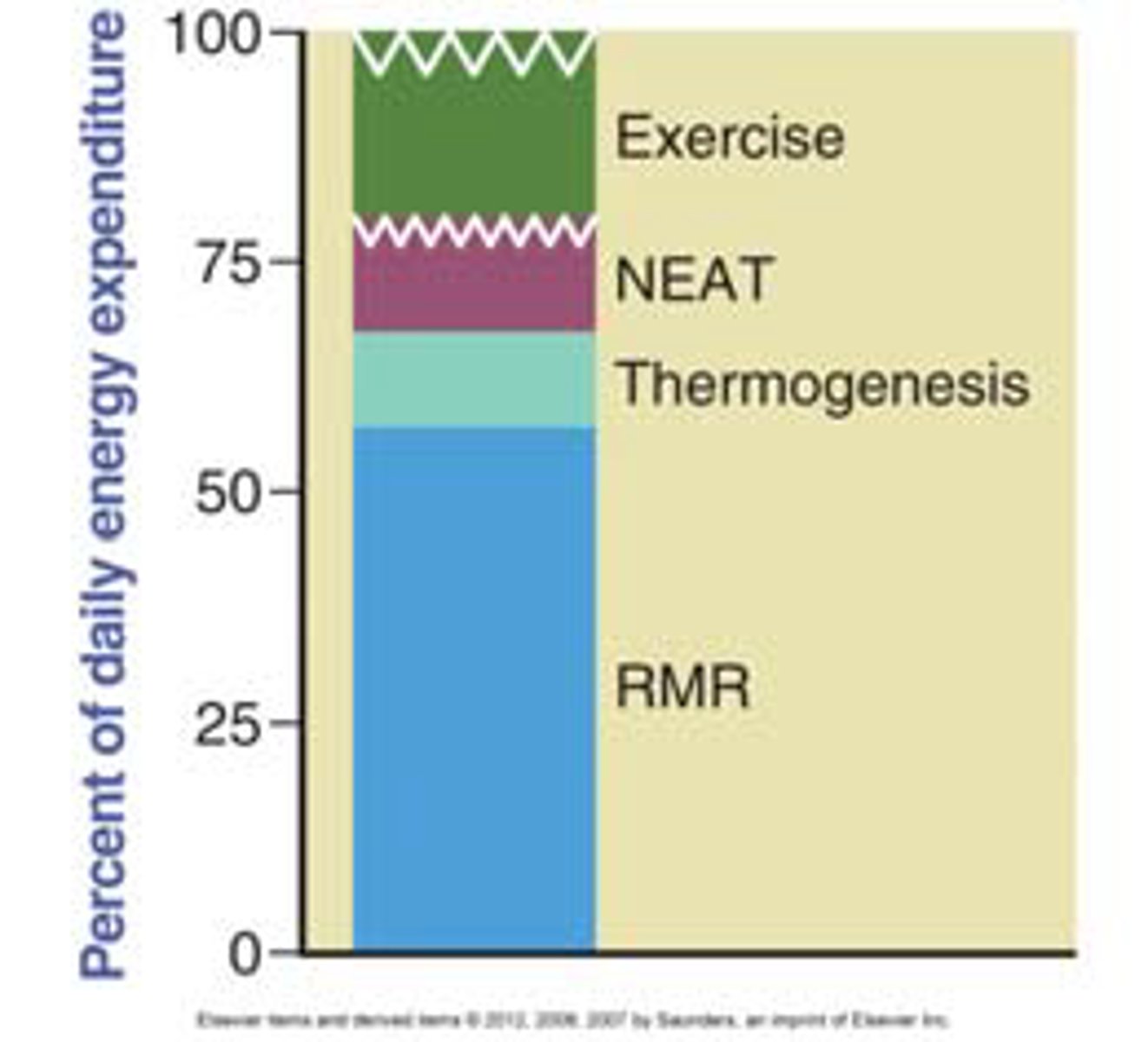
brain signals in a calorie deficit
- strong drive to increase hunger and reduce energy expenditure to restore weight
- brain sends signals that promote hunger
↑ NPY/AgRP (orexigenic, hunger-promoting)
↓ POMC/CART (anorexigenic, satiety)
obesity alters neuro-molecular mechanisms that regulate reward
- in obesity, feelings of reward for pleasurable stimuli are decreased
- upon dietary change/ weight loss, feelings of reward for palatable food are increased, making it harder to resist
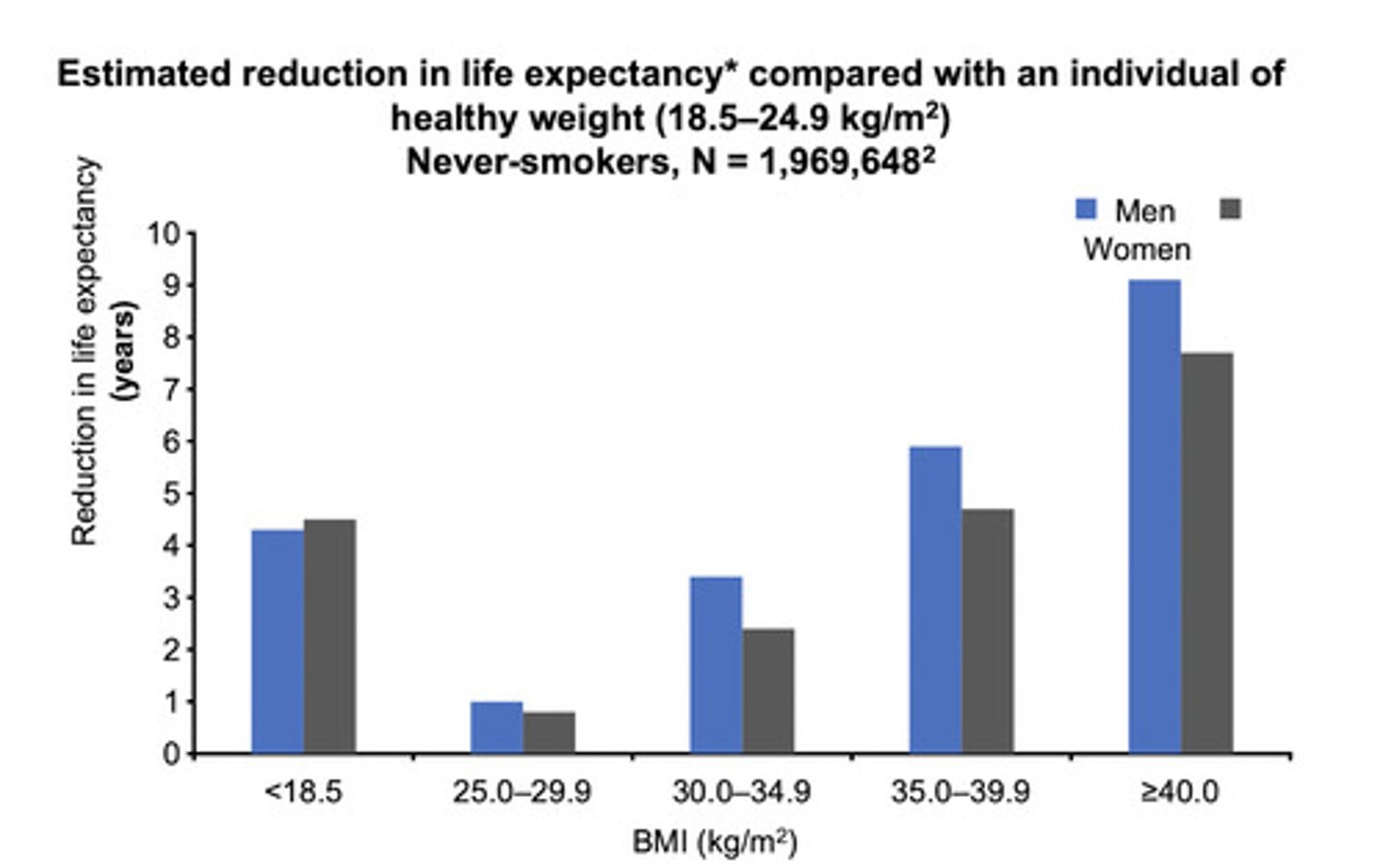
__% increase in mortality is associated with every _ BMI point increase above 25
30%
5
fatty liver
metabolic-dysfunction associated liver disease (MASLD)
- strong correlation with metabolic health and obesity
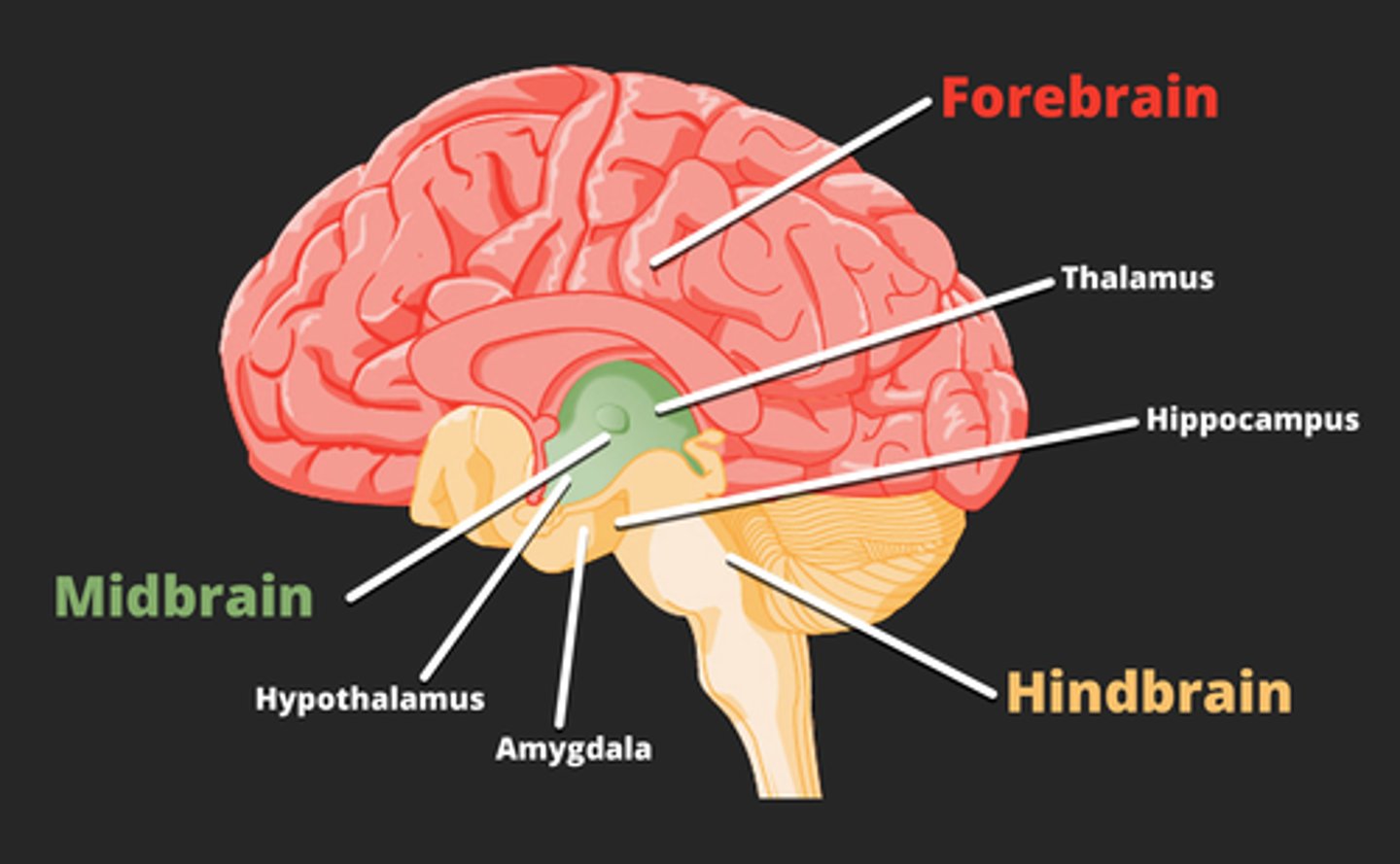
the __ and __ are primary central energy regulating sites that integrate information about blood (hormones, nutrients) and neuronal input (gut vagal afferents)
hypothalamus (POMC/CART)
hindbrain (where vagal afferent nerve terminates, area postrema)
risks for metabolic dysfunction (insulin resistance, dyslipidemia) go up with waist size that is greater than __ cm for women or __ cm for men
women - 88 cm
men - 102 cm
treatment guidelines for obesity
picture
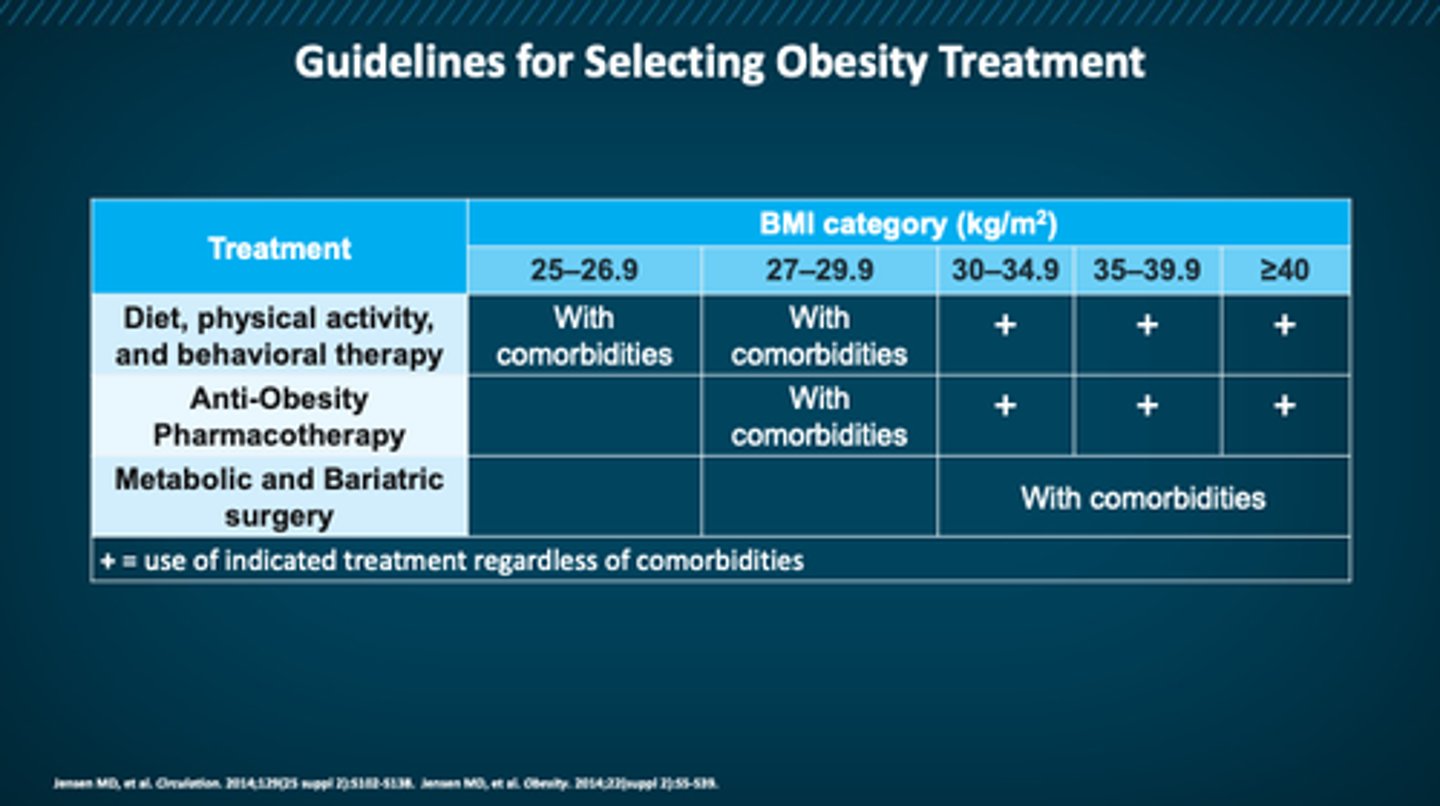
at what BMI is someone a candidate for pharm therapy?
≥30
or ≥27 with comorbidities
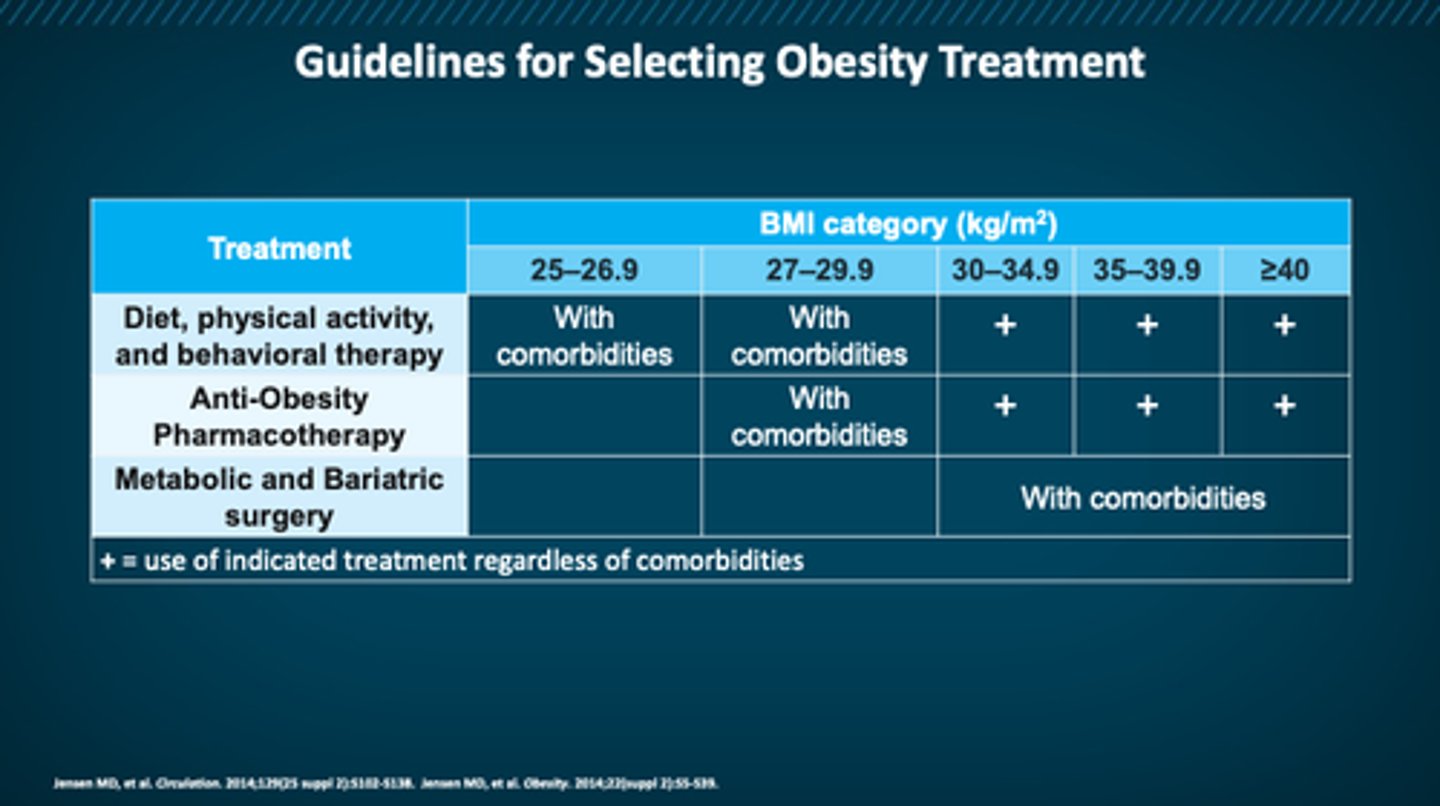
behavioral interventions for obesity
the foundation to obesity treatment
- improved food choices
- increased physical activity
- patient-led health tracking
- sleep hygiene
- stress reduction
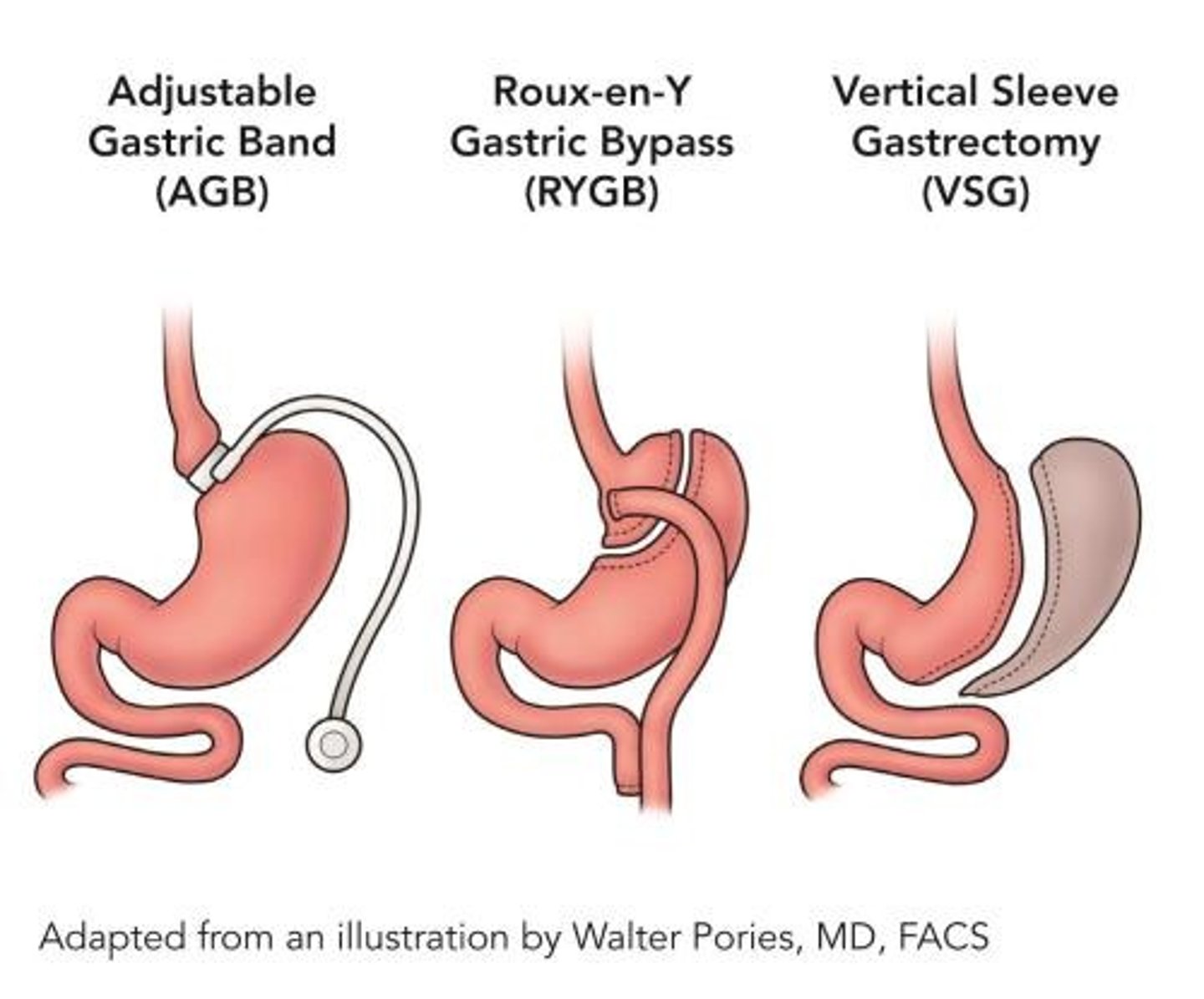
obesity surgery
gastric sleeve: creates a smaller stomach
gastric bypass: creates a pouch stomach + bypasses the duodenum
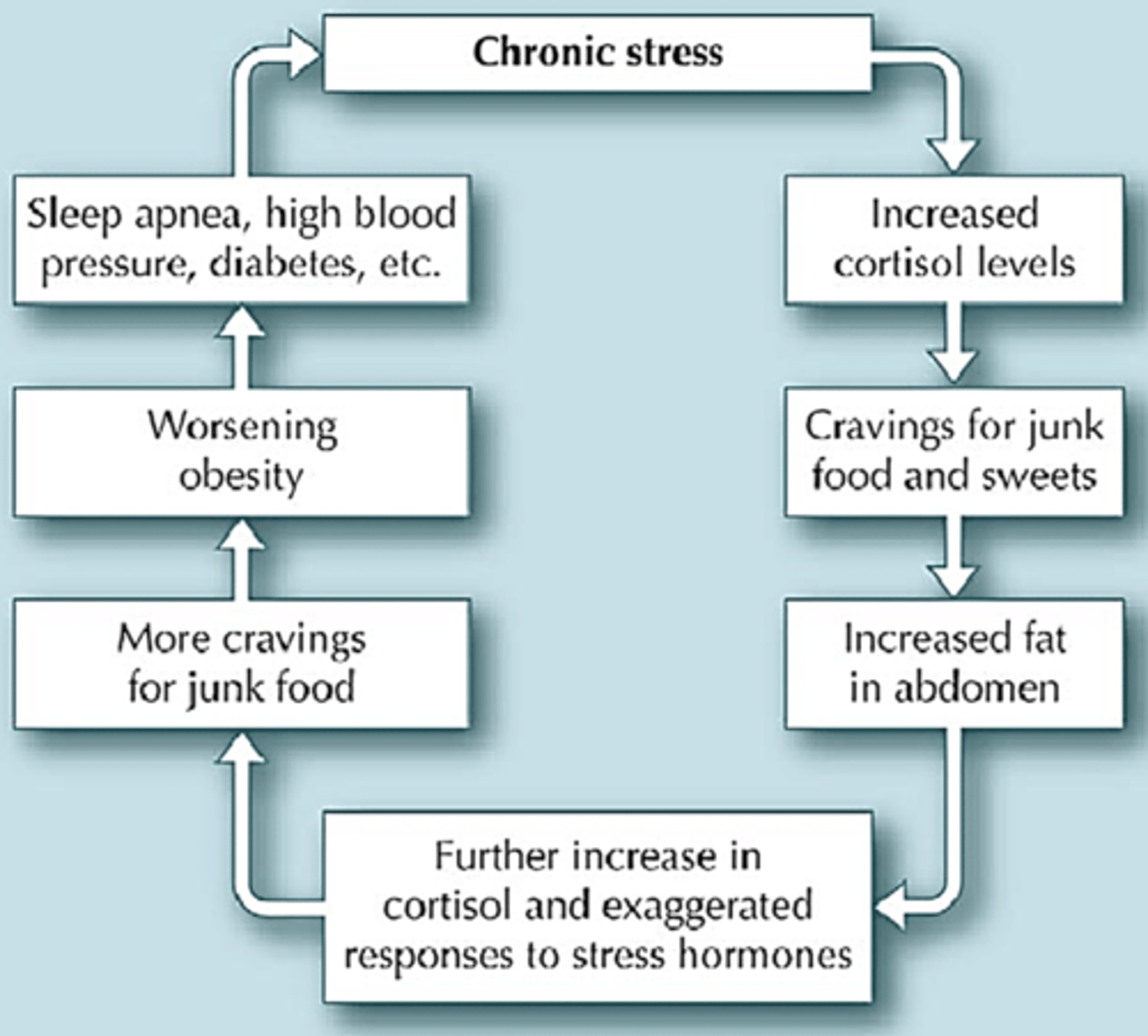
how does stress contribute to obesity?
chronic stress → prolonged cortisol release →
activation of sympathetic NS → change in energy homeostasis → increased fat mass → higher BMI
how does insufficient sleep contribute to obesity?
sleep deprivation:
- ↑ ghrelin ↓ leptin → overeating → ↑ energy intake
- ↑ hedonic signaling → overeating → ↑ energy intake
- ↓ physical activity due to fatigue → ↓ energy expenditure
adult physical activity
- 150+ minutes per week of moderate - vigorous aerobic activity
- muscle strengthening at least 2 days per week
evidence based dieting strategies
1. calorie deficit
2. front load calories in the day (big breakfast, small dinner)
3. adequate fiber
- be sure that patient will adhere to whatever diet changes are put in place
overall treatment approach to obesity
- physical activity: 150 mins/week
- diet: any eating pattern suitable for the patient
- behavioral therapy
- AOMs: for BMI ≥27 + comorbidity OR BMI ≥30
- surgery: for BMI ≥35 comorbidity OR BMI ≥40
FDA approved pharmacotherapies for obesity
- phentermine mono therapy
- orlistat
- phentermine/ topiramate ER
- naltrexone/ bupropion SR
- liraglutide
- semaglutide
- tirzepatide
- setmelanotide
all FDA approved AOMs work by reducing appetite except...
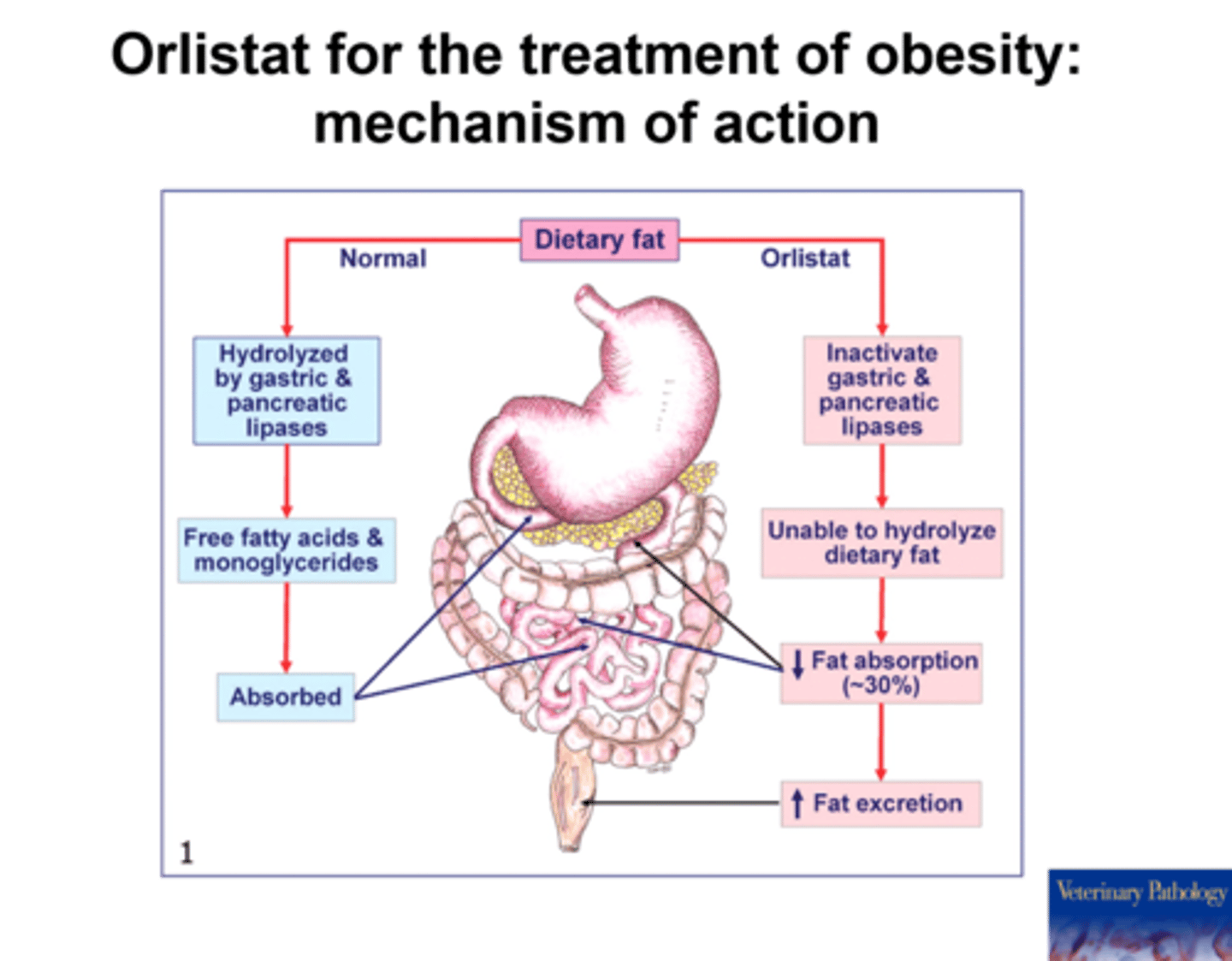
orlistat (Alli)
works by reducing fat absorption