Chemistry Chapter 19 Vocab
- ==Lattice Energy==- the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions under standard conditions.
- ==Enthalpy Change of Atomization ([delta]H[subscript at][superscript naught])==- the enthalpy change when
mole of gaseous atoms is formed from its elements under standard conditions.
==First Electron Affinity ([delta]H[subscript ea1][superscript naught])==- the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms to form 1 mole of gaseous 1- ions under standard conditions.
==Second Electron Affinity ([delta]H[subscript ea2][superscript naught])==- the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous 1- ions to form 1 mole of gaseous 2- ions under standard conditions
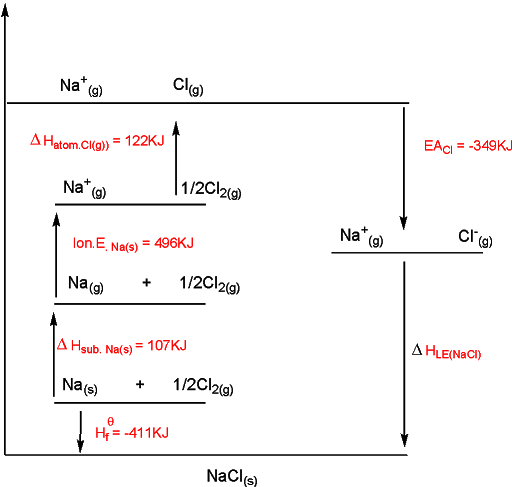
==Born-Haber Cycle==- to draw the cycle, (1) start by putting down elements in their standard state on left-hand side, (2) add the other enthalpy changes in order of steps 1 to 4, (3) complete the cycle by adding the enthalpy change of formation and lattice energy.
step 1: atomize solid to get gas atoms
step 2: convert gas metal to required ion
step 3: atomize the gas nonmetal
step 4: convert gas nonmetal to required ion \n
 6. ==Ion Polarization==- In some cases, the positive charge on the cation in an ionic lattice may attract the electrons in the anion towards the cation, resulting in the distortion of the electron cloud of the anion, the cloud is no longer spherical. (anion is usually bigger than cation)
6. ==Ion Polarization==- In some cases, the positive charge on the cation in an ionic lattice may attract the electrons in the anion towards the cation, resulting in the distortion of the electron cloud of the anion, the cloud is no longer spherical. (anion is usually bigger than cation)  7. ==Polarizing Power==- The ability of a cation to attract cations and distort an anion is called polarizing power of the cation.
7. ==Polarizing Power==- The ability of a cation to attract cations and distort an anion is called polarizing power of the cation.8. ==Enthalpy Change of Solution ([delta]H[subscript sol][superscript naught])==- the energy absorbed or released when 1 mole of an ionic solid dissolves in sufficient water to form a very dilute solution.
9. ==Enthalpy Change of Hydration ([delta]H[subscript had][superscript naught])==- the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form a very dilute solution.
hydration energy. As with lattice energy, hydration energy is a Coulombic energy and thus increases as the ions either increase in charge (i.e., Mg2+ > Na+) or decrease in size (i.e., Na+ > K+).