Chemistry Chapter 19 Vocab
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 4:07 PM on 10/17/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
Lattice Energy
the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions under standard conditions.
2
New cards
Enthalpy Change of Atomization ([delta]H[subscript at][superscript naught])
the enthalpy change when mole of gaseous atoms is formed from its elements under standard conditions.
3
New cards
First Electron Affinity ([delta]H[subscript ea1][superscript naught])
the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms to form 1 mole of gaseous 1- ions under standard conditions.
4
New cards
Second Electron Affinity ([delta]H[subscript ea2][superscript naught])
the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous 1- ions to form 1 mole of gaseous 2- ions under standard conditions
5
New cards
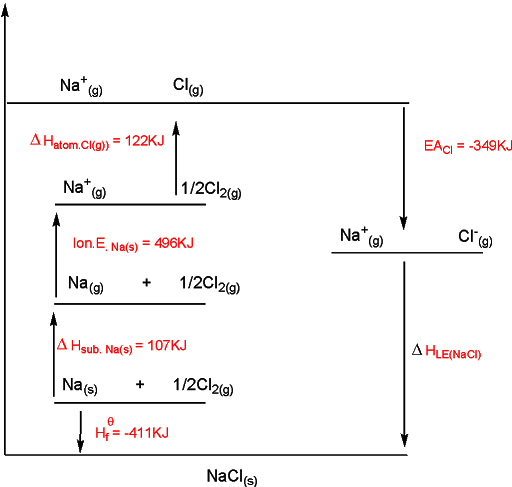
Born-Haber Cycle
to draw the cycle, (1) start by putting down elements in their standard state on left-hand side, (2) add the other enthalpy changes in order of steps 1 to 4, (3) complete the cycle by adding the enthalpy change of formation and lattice energy.
6
New cards
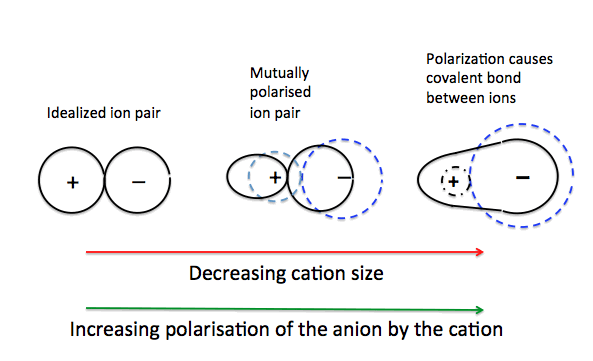
Ion Polarization
In some cases, the positive charge on the cation in an ionic lattice may attract the electrons in the anion towards the cation, resulting in the distortion of the electron cloud of the anion, the cloud is no longer spherical. (usually occurs with small cations)
7
New cards
First Electron Affinity ([delta]H[subscript ea1][superscript naught])- the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms to form 1 mole of gaseous 1
ions under standard conditions
8
New cards
Polarizing Power
The ability of a cation to attract cations and distort an anion is called polarizing power of the cation.
9
New cards
Enthalpy Change of Solution ([delta]H[subscript sol][superscript naught])
the energy absorbed or released when 1 mole of an ionic solid dissolves in sufficient water to form a very dilute solution.
10
New cards
Enthalpy Change of Hydration ([delta]H[subscript had][superscript naught])
the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form a very dilute solution.