Chemistry Core Practical 1: Measure the molar volume of a gas
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Safety
Wear eye protection.
Ensure the delivery tube does not become blocked.
Ethanoic acid will sting if it gets into cuts in the skin.
Equipment
Boiling tube
Stand and clamp
Bung fitted with delivery tube to fit boiling tube
Water bath for gas collection
100 cm³ measuring cylinder
50 cm³ measuring cylinder
Test tube
Mass balance (2 d.p.)
One mol dm-³ ethanoic acid
Powdered calcium carbonate
Diagram
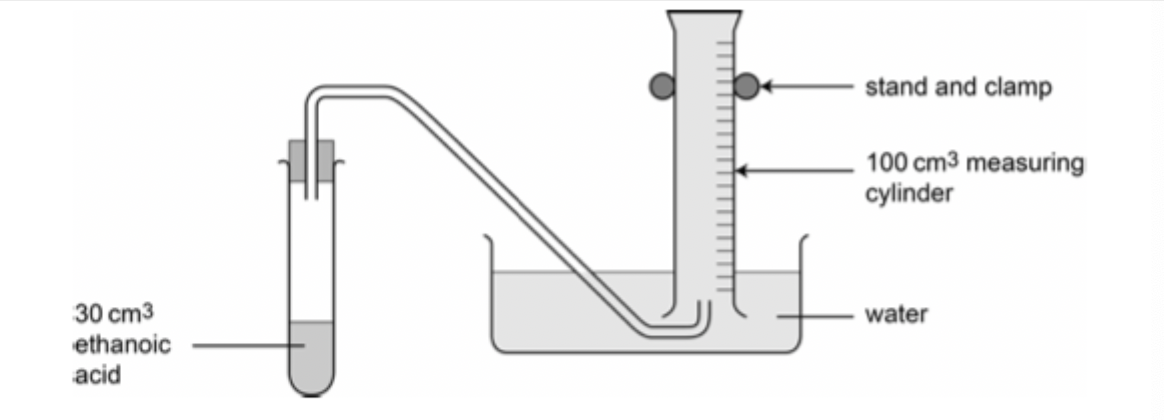
Procedure
Place 30 cm³ of 1 mol dm-³ ethanoic acid in the boiling tube.
Set the apparatus up as shown in the diagram.
Place approximately 0.05g of calcium carbonate in a test tube.
Weigh the test tube and its contents accurately.
Remove the bung from the boiling tube and tip the calcium into the boiling tube. Quickly replace the bung in the boiling tube.
Once the reaction is over, measure the volume of gas collected in the measuring cylinder.
Reweigh the test tube that had contained the calcium carbonate.
Repeat the experiment six more times, increasing the mass of calcium carbonate by about 0.05g each time. Do not exceed 0.40g of calcium carbonate.
Balanced equation of reaction
2CH3COOH + CaCO3 —> Ca(CH3COOH)2 + CO2 +H2O
Why is it more accurate to find the mass of the calcium carbonate used by weighing the test tube with calcium carbonate in, then tipping it out and reweighing the test tube, rather than weighing an empty tube at the start?
This method accounts for any calcium carbonate that might remain stuck to the sides of the test tube and not enter the boiling tube. Therefore, the mass recorded is closer to the true value of mass reacted than only weighing the test tube once.
Identify the major source of error. What change to the procedure/apparatus could be made to eradicate this error?
Some CO2 may be dissolved in the water instead of being collected in the cylinder. Collect the gas in a gas syringe instead.
How can percentage error be reduced?
Increase the measurement size
Decrease the absolute uncertainty by using more precise apparatus
Remove the bung from the boiling tube and tip the calcium into the boiling tube. Quickly replace the bung in the boiling tube.
Explain why Step 5 is the largest source of error in this experiment and suggest how this error could be overcome.
Gas is lost from the boiling tube before the bung is connected, so an unqualified amount of gas is not collected in the measuring cylinder, lowering the measurements relative to the true values
Need a closed system in place before the two reactants are mixed. E.g. calcium carbonate in a small vial placed in the conical flask with the acid, bung is sealed, then apparatus agitated to mix reactants