Electron Structure
1/10
Earn XP
Description and Tags
5.1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
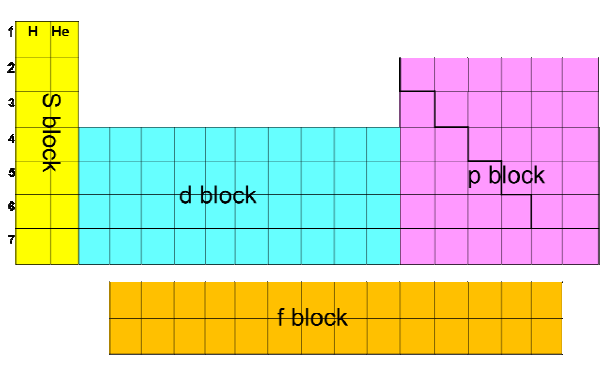
Define sub-shells
Sub-shells are the components of energy levels
What are orbitals
Sub-shells are split into orbitals which hold up to 2 electrons of opposite spin
What are the 4 types of sub-orbitals and how many electrons can each one hold
s- hold 2 electrons
p- hold 6 electrons
d- holds 10 electrons
f- holds 14 electrons
What is the shape of s orbital
Sphere
What is the shape of a p orbital
Dumbell
What are 3 rules of filling orbitals
Orbitals fill in order of increasing energy
4s orbital is of lower energy than 3d
Orbitals with the same energy are occupied singly first
Electrons pair with opposite spins
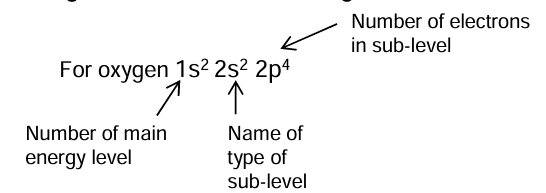
Write and label the electron configuration of oxygen

How are positive ions formed
Positive ions are formed when atoms lose electrons
How are negative ions formed
Negative ions are formed when atom gains electrons
How does formation of ions affect electron configuration
When forming ions, the highest energy sub-shell loses/gain electrons
When losing electrons 4s loses electrons before 3d
How is the periodic table divided into subshells