CU Boulder Chemistry 1113 Exam 1
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms
Mega (M)
1,000,000 or a million times the base unit
10^6
Kilo (k)
1,000 or a thousand times the base unit
10^3
Deci (d)
0.1 or a tenth of the base unit
10^-1
Centi (c)
0.01 or a hundredth of the base unit
10^-2
Milli (n)
0.001 or a thousandth of the base unit
10^-3
Micro (µ)
0.000001 or a millionth of the base unit
10^-6
Nano (n)
0.000000001 or a billionth of the base unit
10^-9
Pico (p)
0.000000000001 or a trillionth of the base unit
10^-12
Accuracy
Refers to the closeness of a measured value to a standard or known value. (How close your measurement is to the one you were supposed to get, the closer the numbers the higher the accuracy)
Precision
Refers to the closeness of two or more measurements to each other. (How close are the numbers you are measuring each time, the closer the more percise)
Significant Figures in Multiplication and Division
The LEAST number of significant figures in any number of the problem determines the number of significant figures in the answer.
Example: 0.2879*1.9870025= 0.5721
^4sig figs ^8sig figs ^4sig figs
Significant Figures in Addition and Subtraction
(1)Count the number of significant figures in the decimal portion of each number in the problem. (The digits to the left of the decimal place are not used to determine the number of decimal places in the final answer.)
2) Add or subtract in the normal fashion.
3) Round the answer to the LEAST number of places in the decimal portion of any number in the problem.
Example: 1.3935+88.7= 90.1
^3 sig figs ^3 ^3 sig figs
Significant Figures when Multiplication / Division is Combined with Addition / Subtraction
First, follow the order of operations (PEMDAS).
If the next operation is in the same group of operations that you just used, then do NOT round off yet.
If the next operation is from the other group, then you must round off that number before moving on to the next operation.
Example: (35.99/22.01234)-0.00234= 1.633
Uncertain Digits
1. Only used when recording measurements.
2. Each measured number has 1 uncertain digit, the end of the number.
Certain Digits
Certain digits + first uncertain digit = the number of significant figures ( certain plus one)
Example: 5.9824
5,9,8, and 2 are certain while 4 is uncertain. this means the accuracy scale is close to the thousandths place rather than the ten-thousandths place.
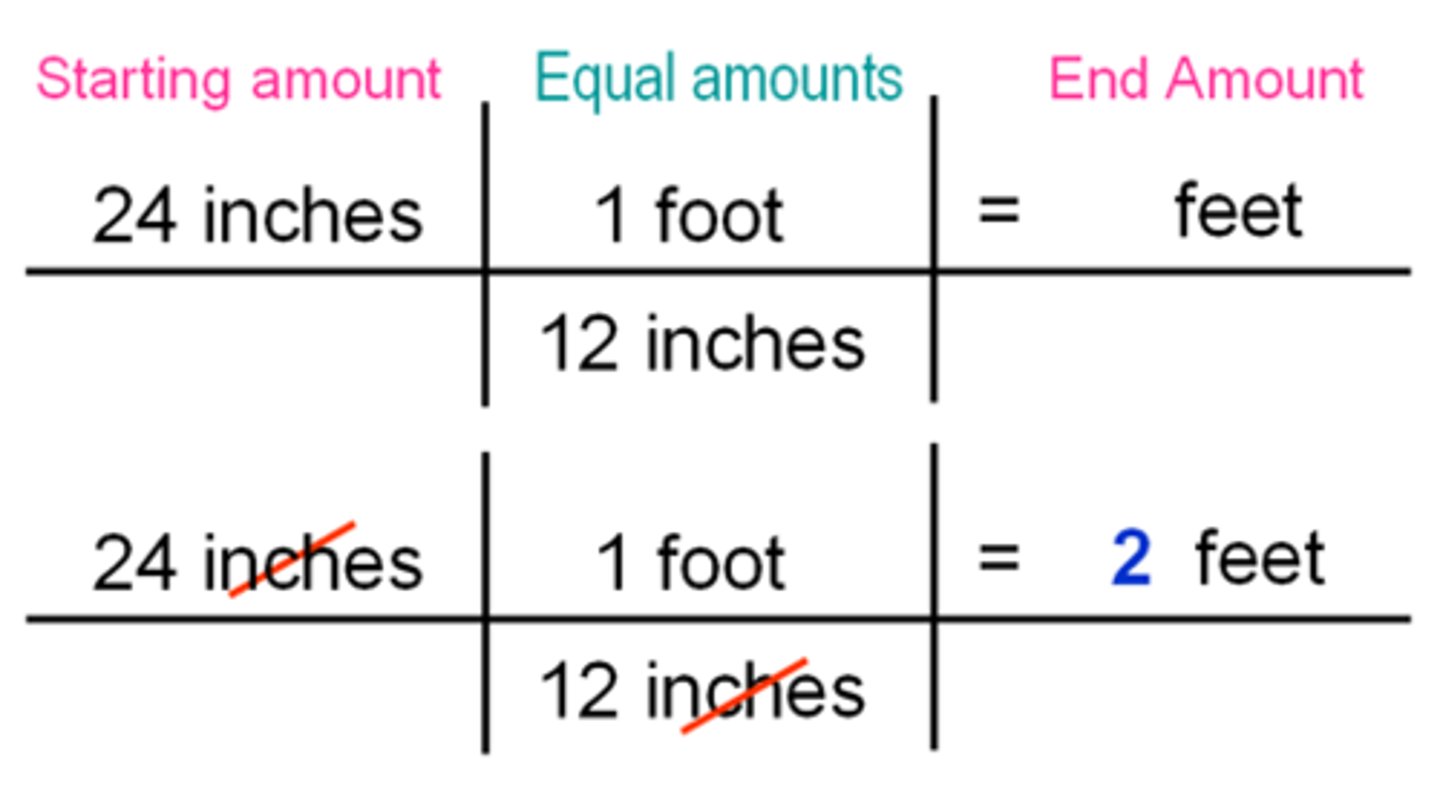
Dimensional Analysis
A problem-solving method that uses the fact that any number or expression can be multiplied by one without changing its value. Unit factors may be made from any two terms that describe the same or equivalent "amounts" of what we are interested in. For example, we know that
1 inch = 2.54 centimeters.
Order of Measurements Based on Size (largest to smallest)
Mega, kilo, deci, centi, milli, micro, nano, and pico
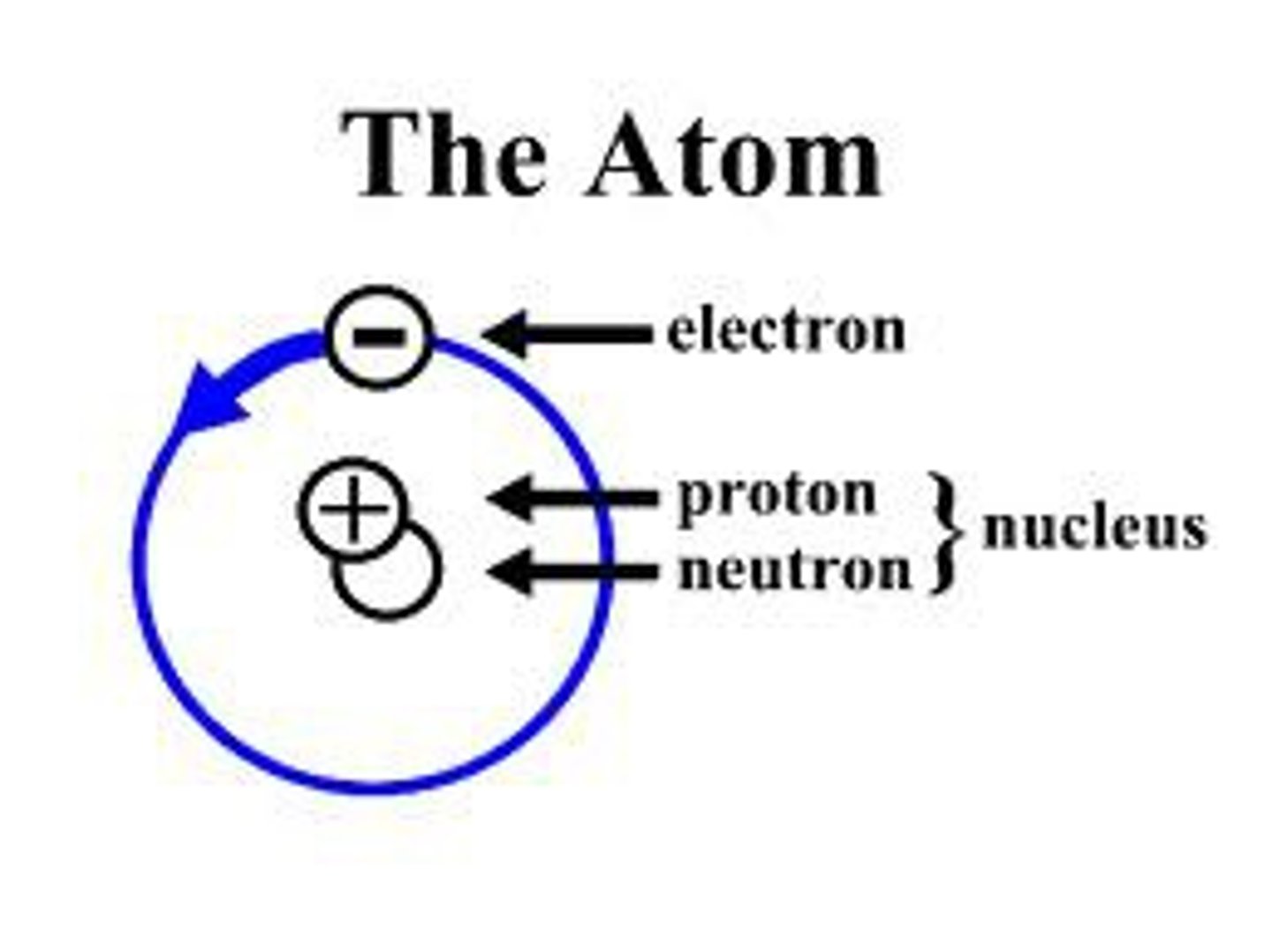
Atoms
The basic unit of a chemical element, a unit of matter.
Elements
A chemical element is a substance that cannot be broken down by chemical means. An element is composed of atoms that have the same atomic number, that is, each atom has the same number of protons in its nucleus as all other atoms of that element.
Substances
A substance is matter which has a specific composition and specific properties. Every pure element is a substance. Every pure compound is a substance.
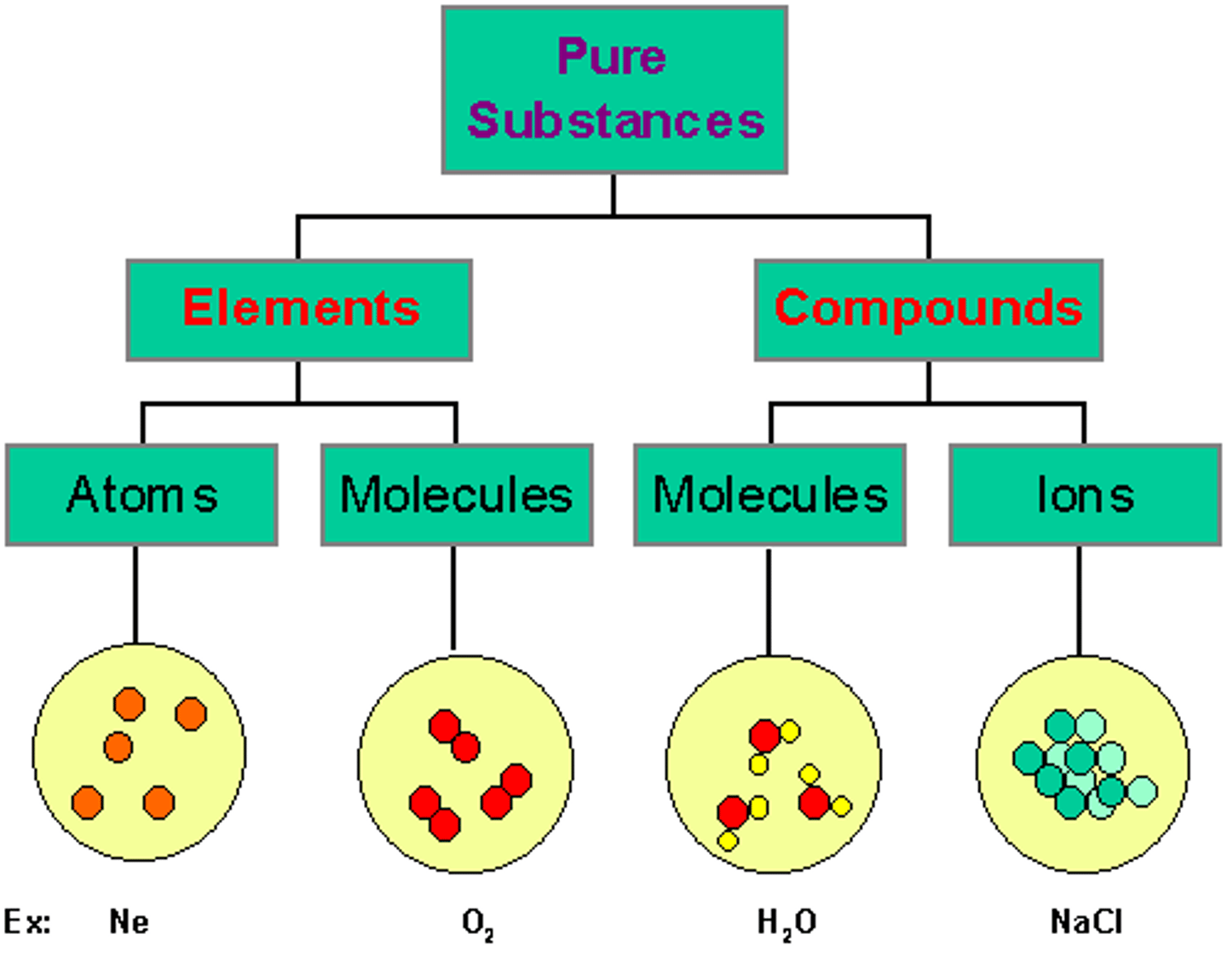
Compounds
A substance formed when two or more chemical elements are chemically bonded together.
Mixtures
A combination of two or more pure substances in which each pure substance retains its individual chemical properties. Mixtures can be composed of solids, liquids, or gases.
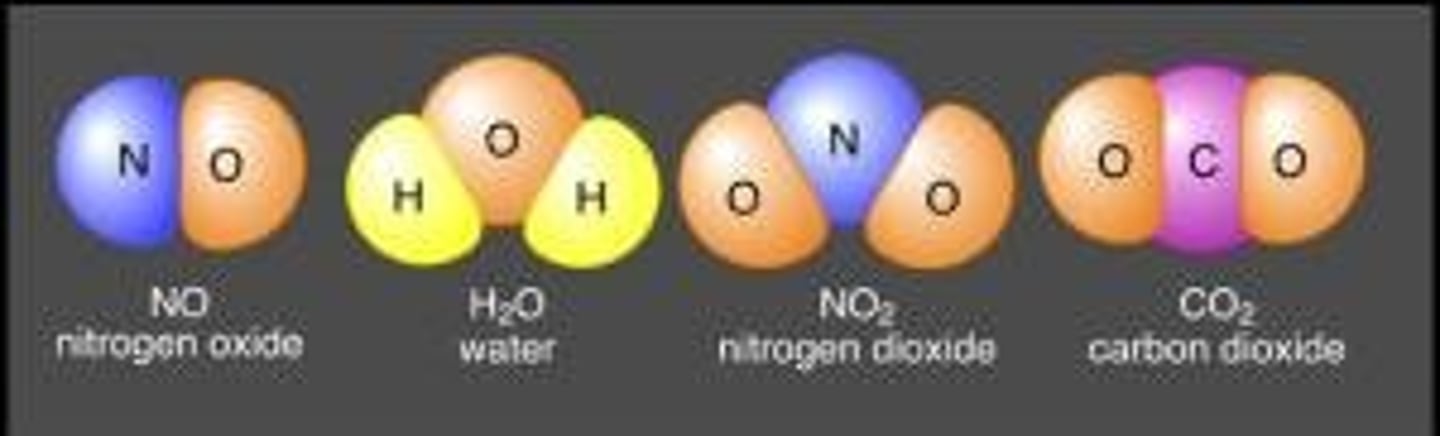
Molecules
Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms.
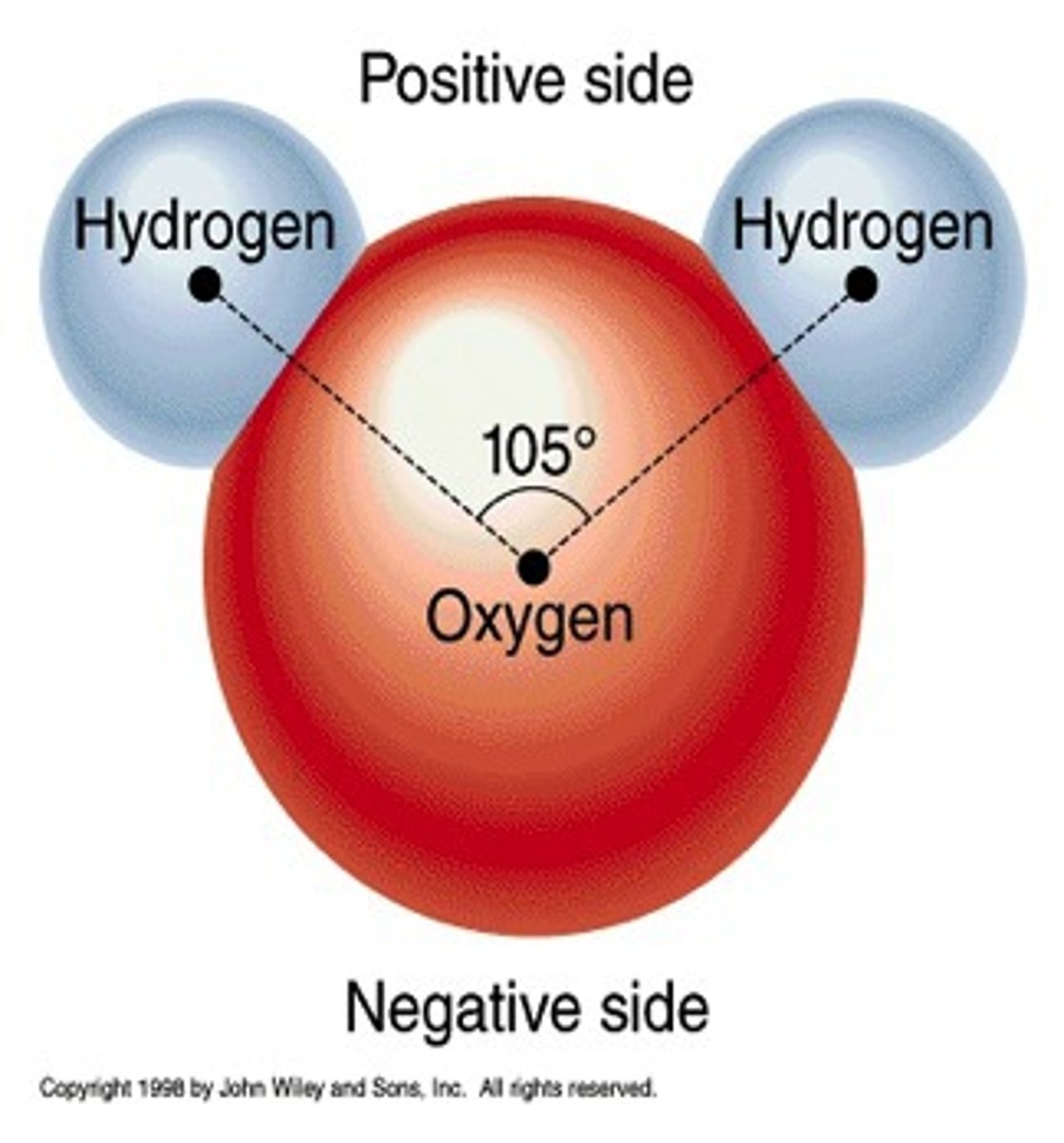
The Postulates of Dalton's Atomic Theory
1.Matter is composed of very tiny or microscopic particles called "Atom".
2.Atom is an indivisible particle.
3.Atom can neither be created nor it is destroyed.
4.Atoms of an element are identical in size, shape, mass and in other properties.
5.Atoms of different elements are different in their properties.
6.Atoms combine with each other in small whole numbers.
7.All chemical reactions are due to combination or separation of atoms.
Number 2, 3, 4 and 6 are all proven to be false today.
Why they are false:
2. Atom can be divided into a number of sub-atomic particles such as electrons, protons, and neutrons etc.
3.Atoms of an element may be different in their masses.
4.All compounds do not have small number of atoms.
6.Atom can be destroyed by fission process in Atom bomb and Nuclear reactor
The Fundamental Laws of Matter
1.Law of Conservation of Mass: the total mass of the products will always be equal to the total mass of the reactants in any chemical reaction
2.Law of Constant Composition: Chemists always use the molecular formula, if there is one, in preference to an empirical formula.
3.Law of Multiple Proportions: The law of multiple proportions of elements in compounds is a consequence of the atomic theory of matter. The compounds CuO and Cu2O have compositions in which the ratios of the elements are 1:1 and 2:1 respectively. Since atoms are indivisible, a compound can have only integral numbers of atoms of each type in its molecules.
J.J. Thomson
Performs experiments with Cathode Ray Tubes (CRT) and discovers
the electron.
Ernest Rutherford
Discovers the nucleus of the atom and demonstrates that the
Plum Pudding Model is incorrect. His experiment is called the Gold Foil Experiment (or
Geiger-Marsden experiment).
Atom Notation
or ca-40 where 40 is the elements atomic mass.
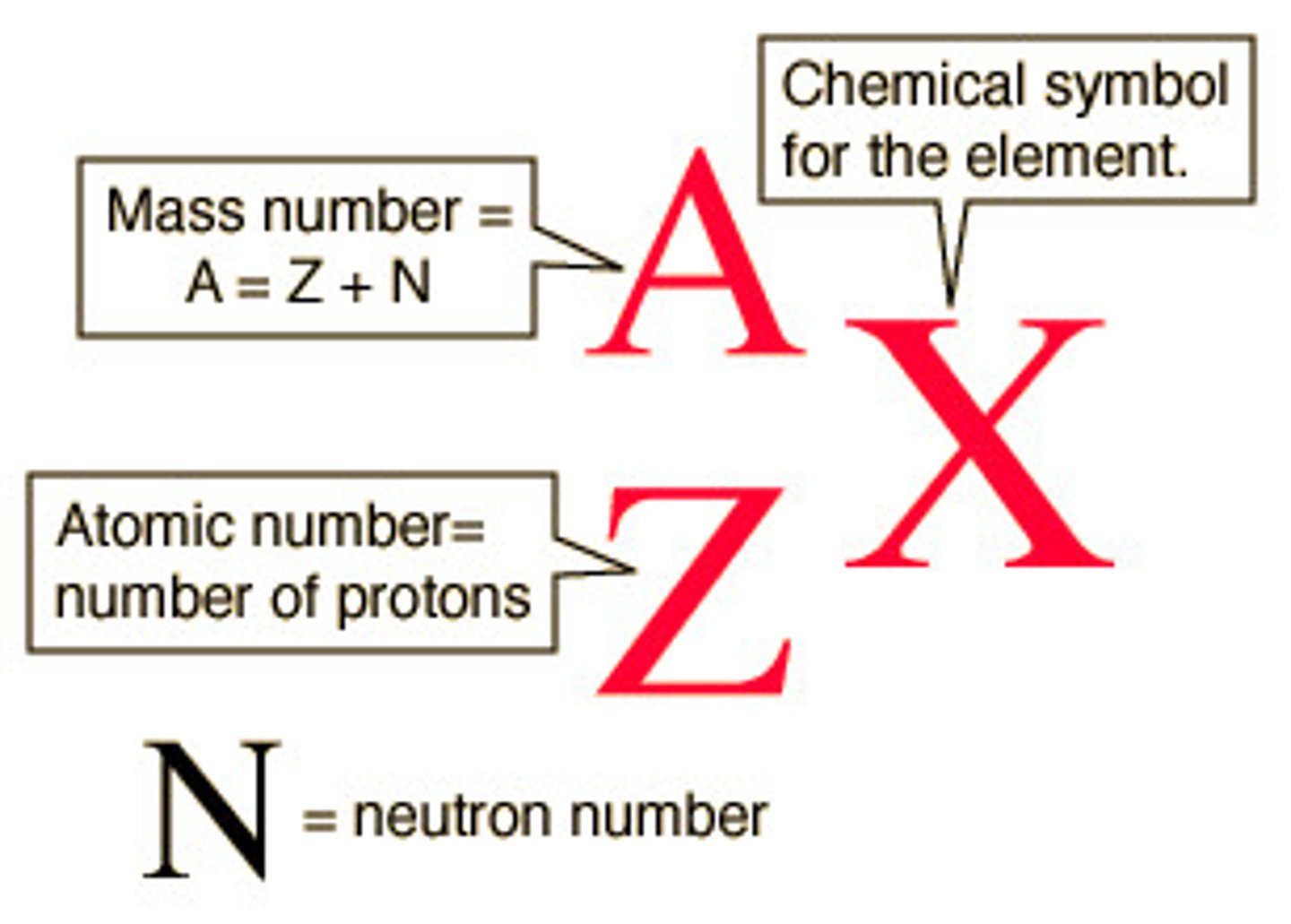
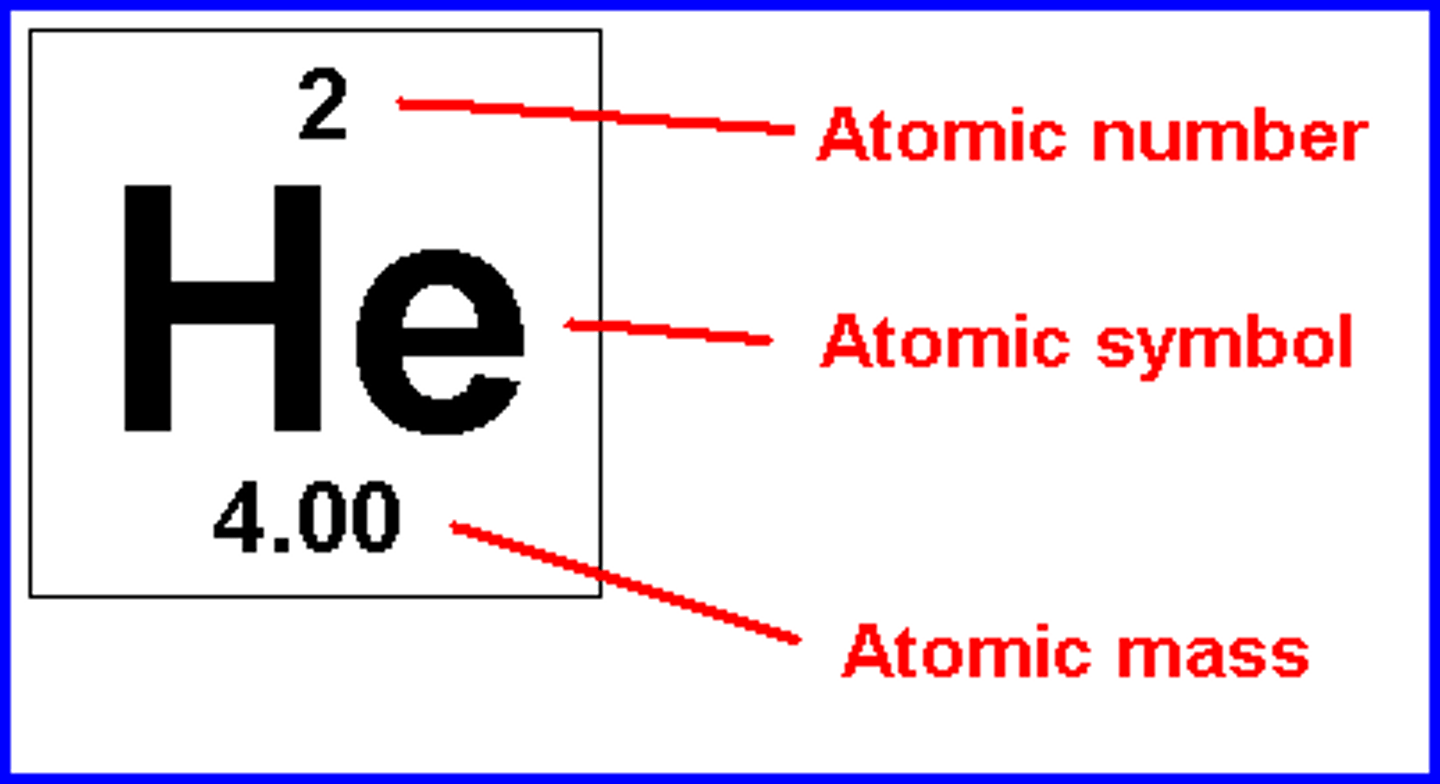
Determining Amounts From Element
Protons: Upper number
Neutrons: Round the atomic weight to the nearest whole number and subtract protons
Electrons: Upper number
Mass Number: below element
Charged v. Not Ions: Transferred by electrons, determine based on periodic table.
Determining Charge of Elements
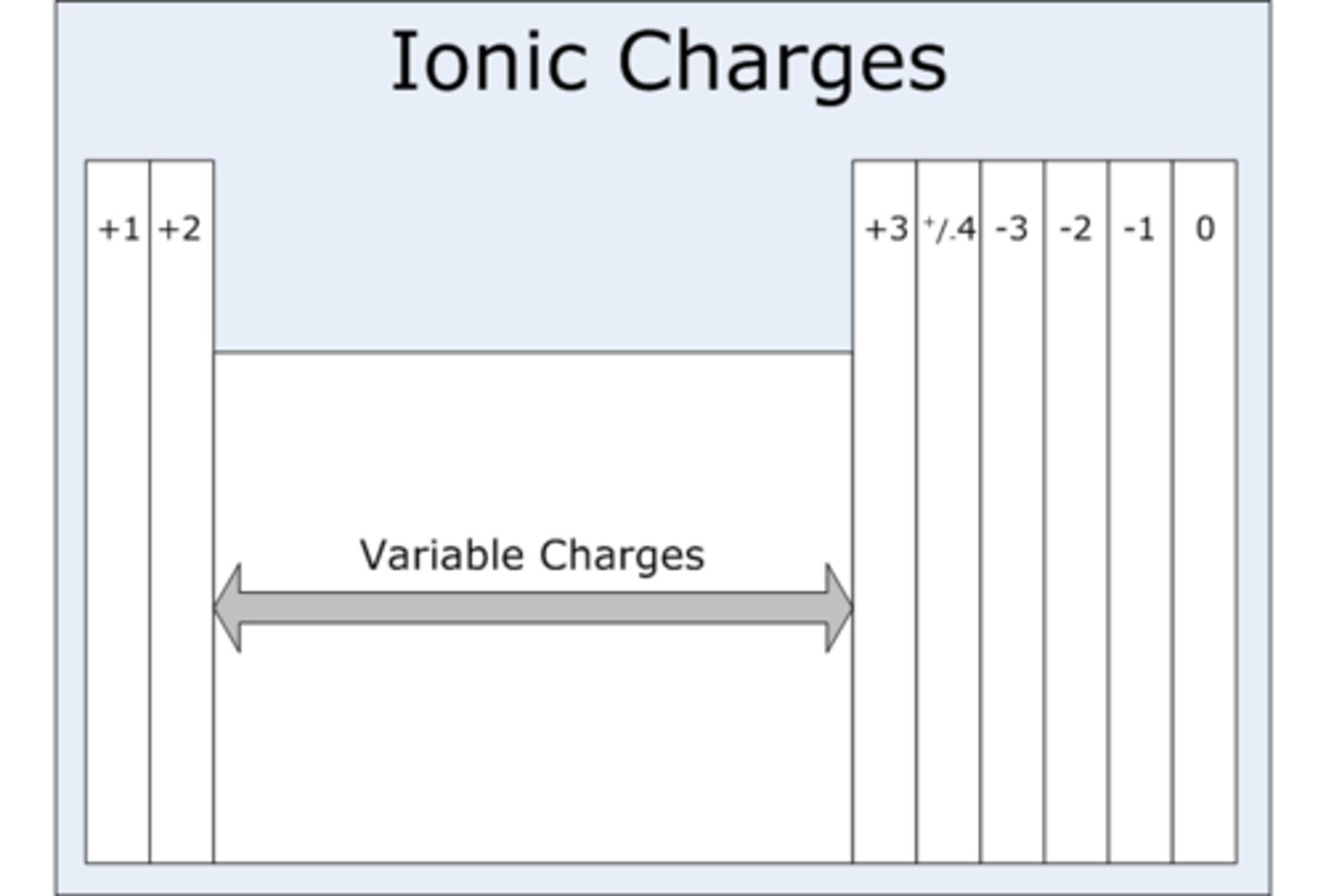
Isotopes
In a given element, the number of neutrons can be different from each other, while the number of protons is not. So isotopes have different numbers of neutrons from the most common element.
Calculating average mass of isotopes
Relative abundance isotope 1(mass of isotope 1) + Relative abundance isotope 2(mass of isotope 2) = weighted average atomic mass

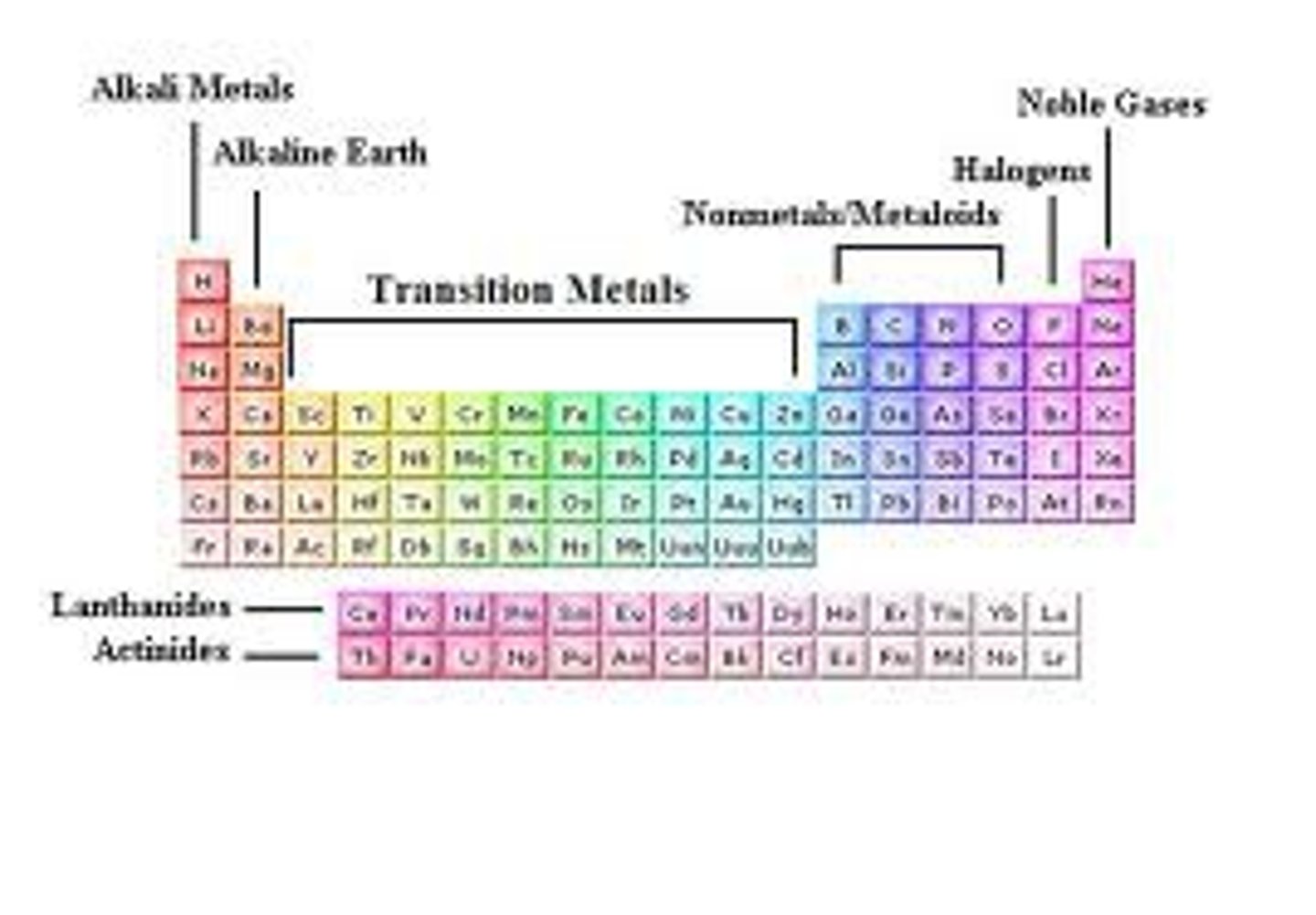
Groups of the Periodic Table
Diatomic Elements
H,N,O,F,Cl,Br,I
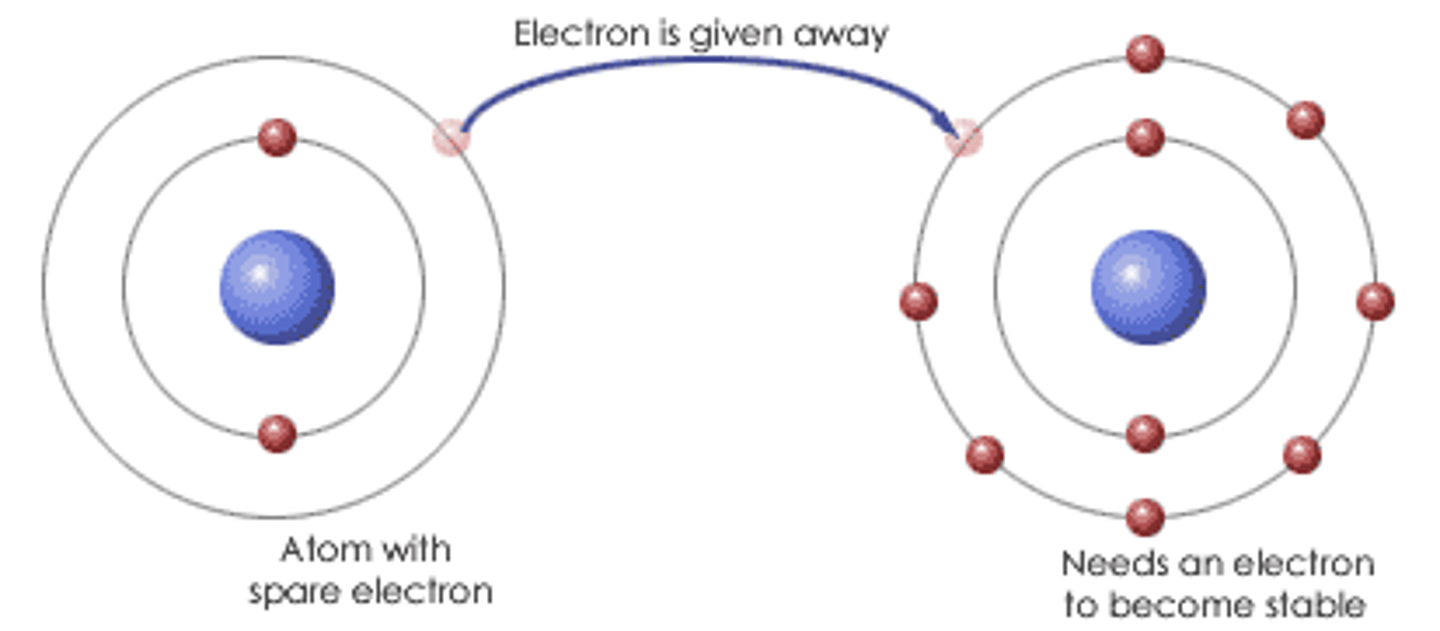
Ionic
Bonding between two metal or a metal and a non metal.
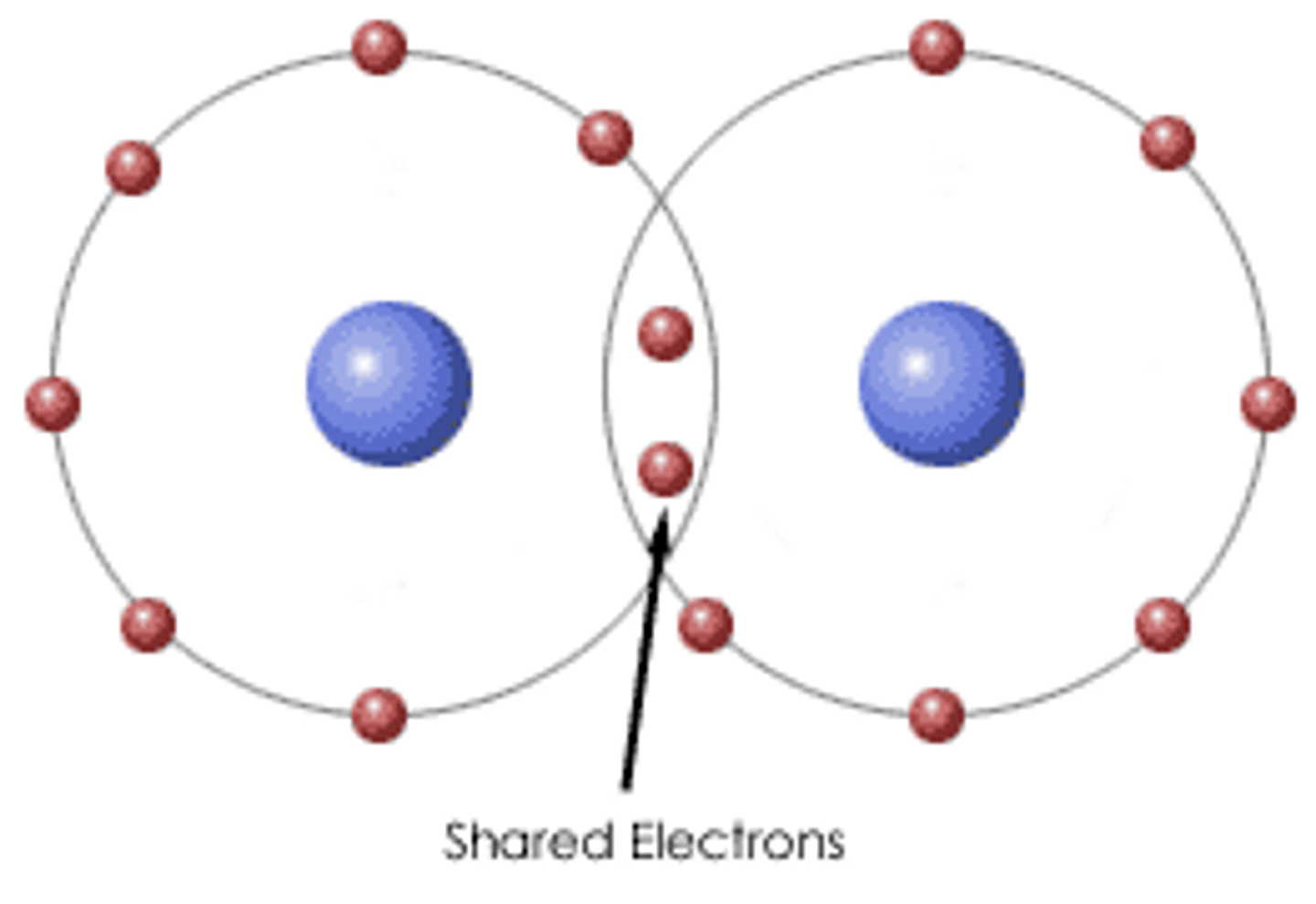
Molecular/Covalent
Covalent bonding is a form of chemical bonding between two nonmetallic atoms
Hydronium
H3O+
Ammonium
NH4+
Hydroxide
OH-
Cyanide
CN-
Nitrate
NO3-
Nitrite
NO2-
Carbonate
(CO3)2-
Hydrogen Carbonate
HCO3-
Sulfate
(SO4)2-
Sulfite
(SO3)2-
Phosphate
(PO4)3-
Hydrogen phosphate
(HPO4)2-
Dihydrogen phosphate
H2PO4-
Acetate
C2H3O2-
Permanganate
MnO4-
Chromate
CrO4(2-)
Dichromate
Cr2O7(2-)
Perchlorate
ClO4-
Chlorate
ClO3-
Chlorite
ClO2-
Hypochlorite
ClO-
Oxoanions Name Change
-=hypo...ite
2-=...ite
3-=...ate
4-=per...ate
Empirical Formula
A formula giving the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.
1.Start with the number of grams of each element, given in the problem.
If percentages are given, assume that the total mass is 100 grams so that
the mass of each element = the percent given.
2.Convert the mass of each element to moles using the molar mass from the periodic table.
3.Divide each mole value by the smallest number of moles calculated.
Round to the nearest whole number. This is the mole ratio of the elements and is represented by subscripts in the empirical formula.
4.If the number is too far to round (x.1 ~ x.9), then multiply each solution by the same factor to get the lowest whole number multiple.
e.g. If one solution is 1.5, then multiply each solution in the problem by 2 to get 3.
e.g. If one solution is 1.25, then multiply each solution in the problem by 4 to get 5.
Molecular Formula
A formula giving the number of atoms of each of the elements present in one molecule of a specific compound.
1. Divide the molecular weight by the empirical units ( the mass of the emperical formula's elements) to find how many to multiply the empirical formula by.
Structural Formula
A formula that shows the arrangement of atoms in the molecule of a compound.
Moles to Mass/ Mass to Moles
* the molar mass/ divided by the molar mass
Moles to Atoms/ Atoms to Moles
* avagadros number/ divided by avagadros number