MIMM214 midterm notes
1/267
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
268 Terms
Intracellular vs extracellular pathogen examples
Intracellular: Virus
Extracellular: Bacteria
Intracellular vs extracellular pathogens are processed differently + generate different types of responses
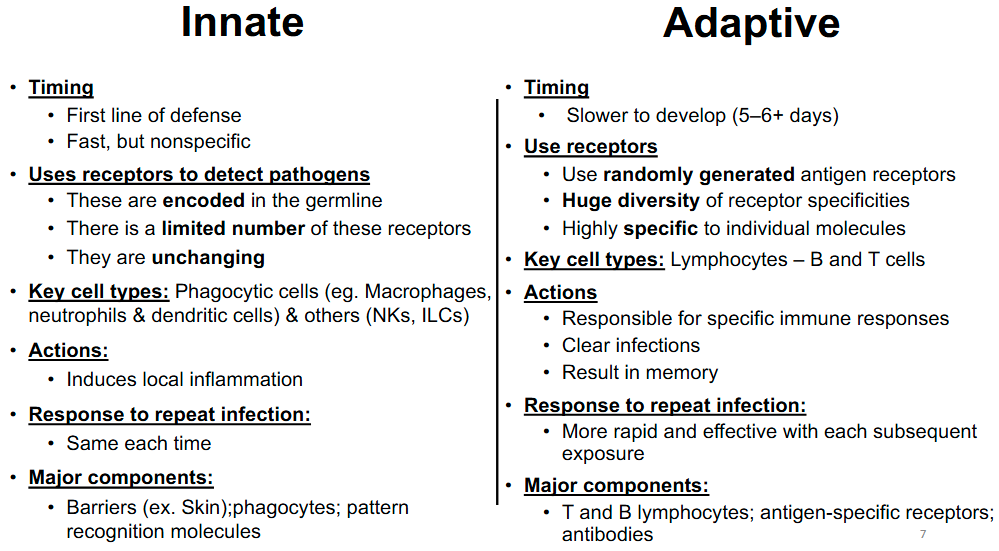
Innate vs Adaptive immune response

Primary lymphoid organs

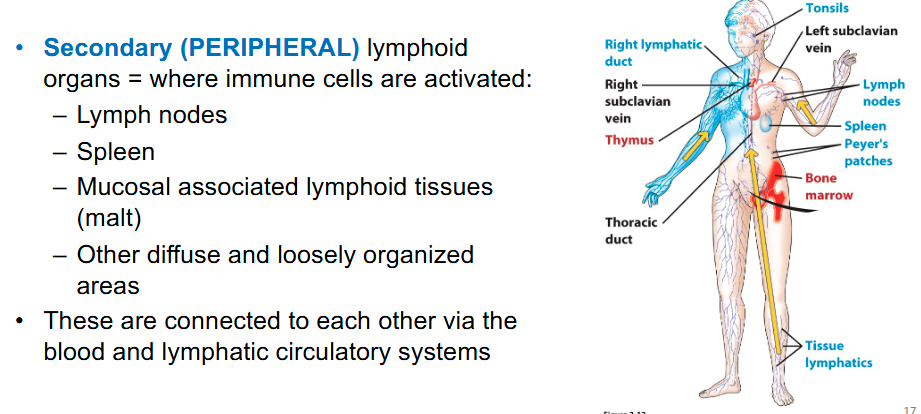
Secondary lymphoid organs
Where are leucocytes (immune cells) produced?
Bone marrow by hematopoiesis
Hematopoiesis
Process by which HSCs differentiate into mature blood cells
- Occurs in the bone marrow
Pluripotent stem cells
stem cells can generate almost every specialized cell type in an organism
Multipotent stem cells
stem cells generate various cell types in a family of related cells
What immune cells does the common lymphoid progenitor give rise to?
B cells, T cells, dendritic cells and NK cells (ILCs)
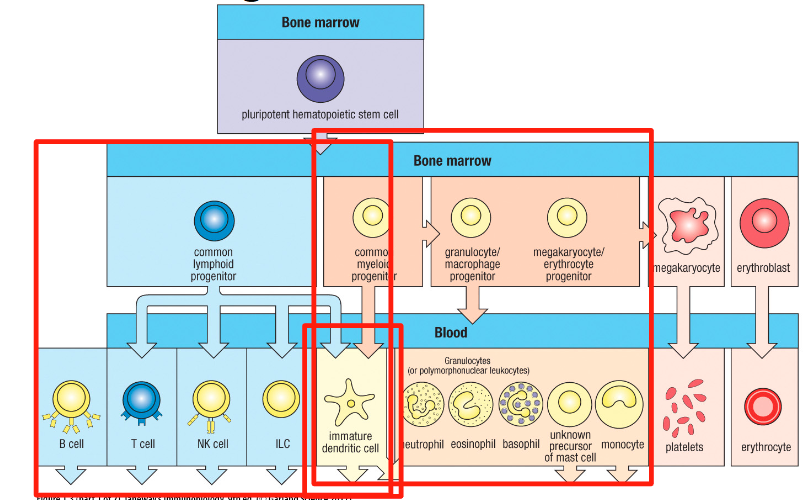
Myeloid lineage gives rise to?
& Dendritic cells
Monocytes function?
Migrate into tissues and differentiate into macrophages―function to repair/remodel, destroy pathogens, present antigens (what induces an immune response)
Macrophages, immature dendritic cells and neutrophils function?
Specialized for phagocytosis (cellular uptake by engulfment)
Macrophages can also present antigens to T cell
Immature dendritic cells capture antigen, then mature and migrate out of that location to another to present antigen to T cells
What is the most potent APC for activating T-cells?
Dendritic cells are the most potent antigen-presenting cells for activating naïve T cells
Macrophages do it too but does it worse
Dendritic cell function?
• Involved in detecting infection
• Potent antigen presenting cells
• Activate adaptive immunity
Bridge between innate and adaptive immune responses
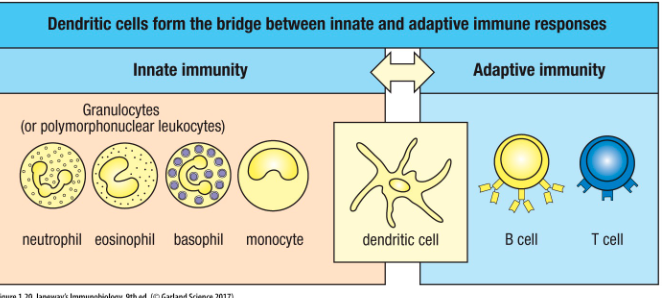
What are the lymphoid cells responsible for adaptive immunity?
T and B cells are responsible for adaptive immunity
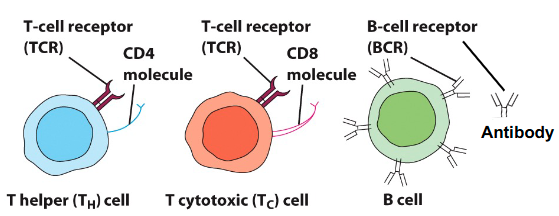
How is the specificity of B and T cells determined?
• Specificity is determined by expression of receptors on cell surface
• B cells - B cell receptor (BCR)
– Can be bound to membrane or secreted as antibodies
• T cells – T cell receptor (TCR)
– Membrane bound
Key types of molecules in the immune system
How are immune responses generated against?
Immune responses are generated against key specific components known as antigens
What are antigens?
• An antigen (Ag) is any specific molecule that can trigger an immune response
• It can be a protein (most), nucleic acid, polysaccharide, lipid, organic chemicals, drugs.
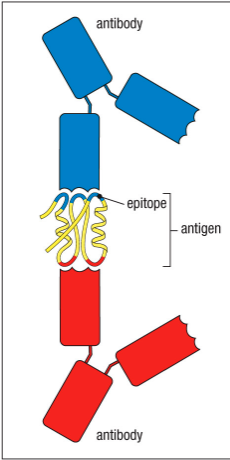
Epitope
The specific portion of an Ag that is recognized by a receptor on an immune cell is called epitope
Innate immunity vs adaptive immunity
All innate cells will have the same receptors and are non specific
Pattern recognition receptors (PRRs)
Immune cells express PRR:
Provide an initial discrimination between self and non-self and recognize broad categories of molecules that are commonly found in pathogens (PAMPs)
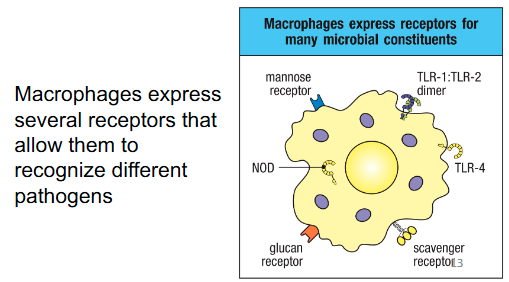
Pathogen-associated molecular patterns (PAMPs)
Common foreign structures that characterize whole groups of pathogens (part of many microorganisms but not of the host body’s own cells)
Same as MAMPs
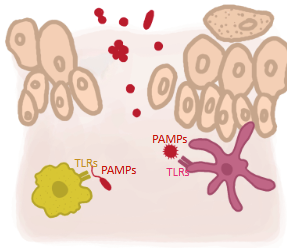
Process of Innate cell activation & local inflammation
• Activation of PRRs on cells (ex. Macrophages) can directly induce effector functions in these cells (ex. Phagocytosis)
• These cells amplify the immune response by the production of inflammatory mediators
- Cytokines and chemokines
• Dendritic cells (immune cells) also gets activated
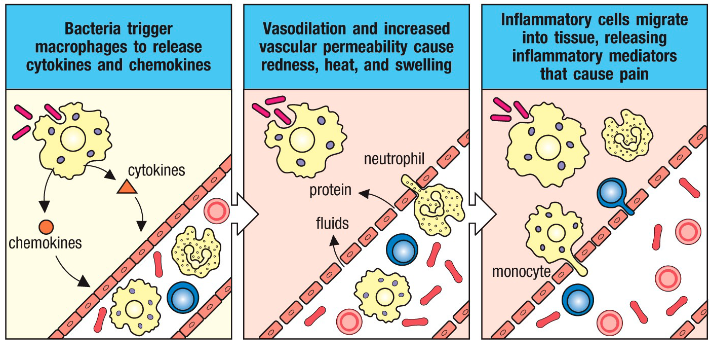
Dendritic cells (DCs) function in pathogen detection (innate immunity)
• Detect pathogens (PAMPs) using receptors (PRRs)
• This detection leads to DC activation
• DCs then link innate to adaptive immunity by being potent APCs
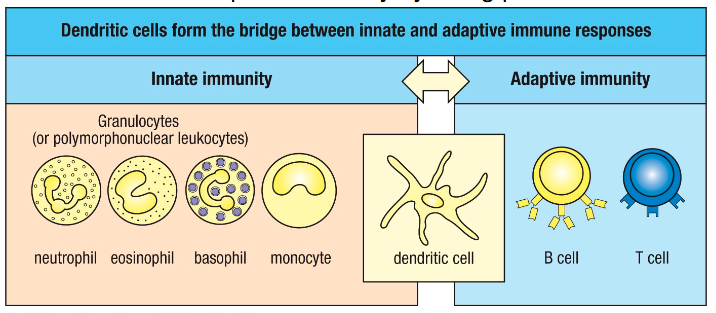
How do dendritic cells link innate to adaptive immunity?
DCs travel from site of infection to local secondary lymphoid tissue (lymph node).
In secondary lymphoid tissue (lymph node), they interact and activate T cells
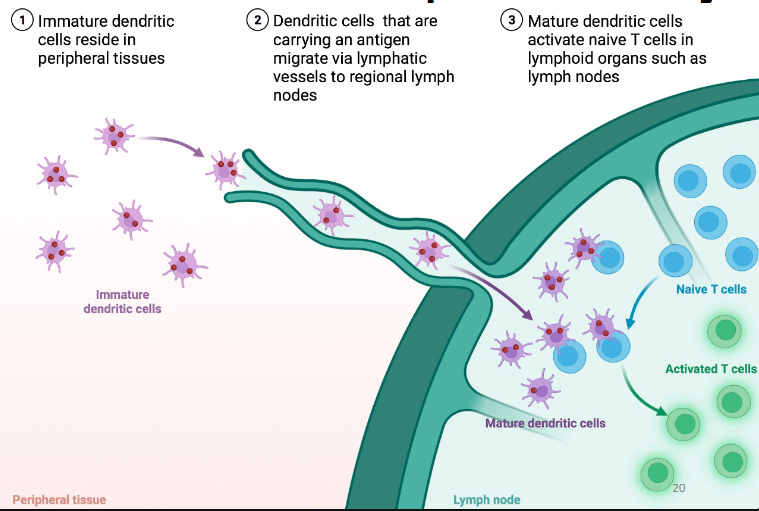
Where do T & B cell activation occur?
T & B cell activation occurs in the lymph node CD4 (by dendritic cells)
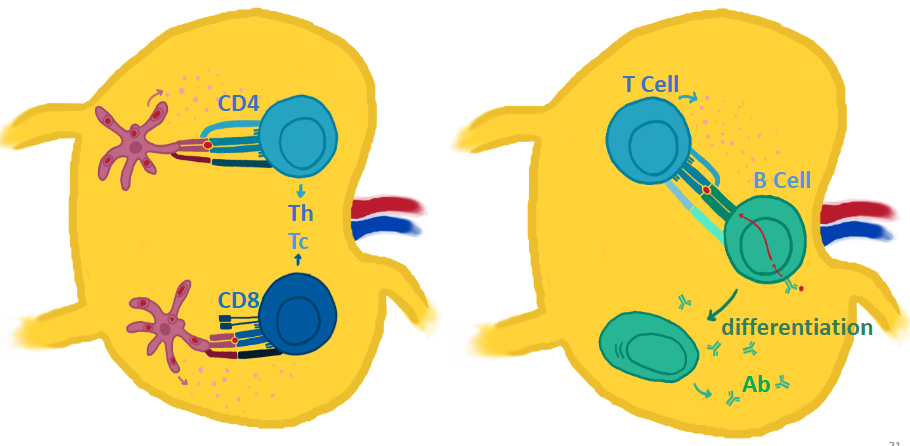
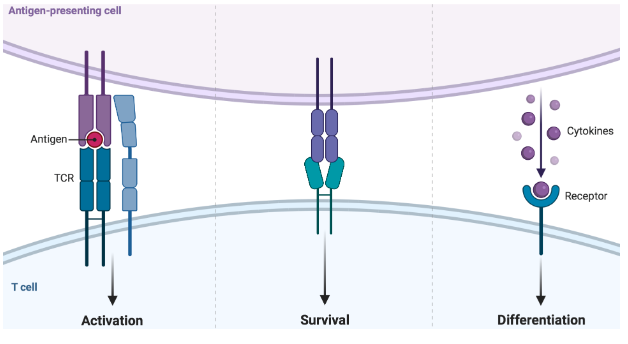
What requirements are needed to activate T cells?
• Antigen-presenting cells (APCs) activate T cells
• Activation happens through 3 signals
- Through interaction of specific molecules (receptors and cytokines)
• Happens in peripheral lymphoid tissue (lymph node)
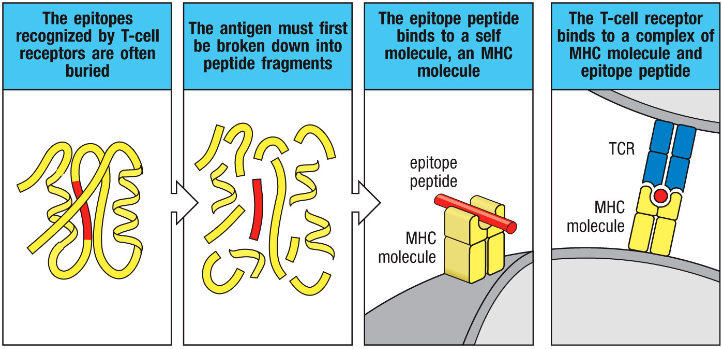
How is the epitope of an antigen acquired and presented to T cells?
• The epitope of an antigen (bacteria) can be a piece of peptide buried within a protein
• Antigen/epitope is presented using a specific molecule (Major Histocompatibility Complex - MHC) on APCs, which interacts with TCR
T-cells will ONLY bind to the epitope if it’s presented on MHC
What are antigen-specific cells? Where are they activated?
• Antigen-specific cells are activated in the secondary lymphoid tissues (ex. Lymph nodes)
• These cells are:
- T cells
- B cells
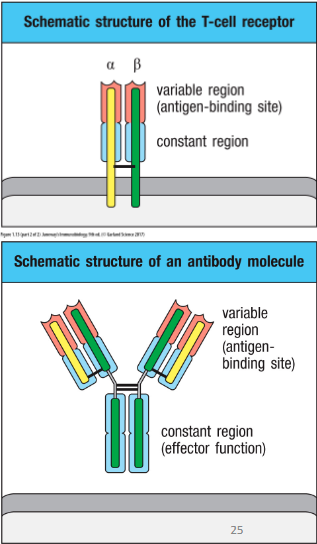
How are B & T cell antigen specificity determined?
• Their antigen specificity is determined by their
receptors
- T cells
→ T cell receptor (TCR)
- B cells
→ B cell receptor (BCR aka antibody aka immunoglobulin)
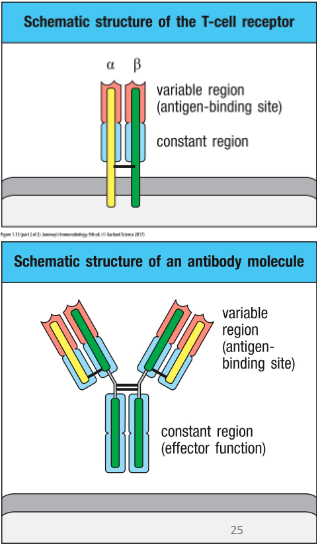
How are antibodies made?
• Antibodies (Abs): secreted immunoglobulin (Ig) molecules
- Made by B lymphocytes and its progeny plasma cells
- Bind Antigens (Ag)
Can two antibodies recognize different epitopes on the same antigen?
Two Abs can recognize different epitopes on the same antigen
B vs T cells
B cells arise and mature in the bone marrow, while T cells arise in the bone marrow but mature in the thymus
BCR can be membrane-bound or secreted (Abs). TCR only exits as membrane-bound
TCR only recognize antigens if presented on MHC
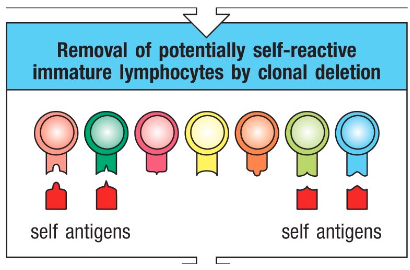
Self-reactive T & B cells
• Rearrangement and editing of the genomic DNA of antigen receptors are random, and sometimes, TCRs and BCRs can be specific to self-antigens
• During development, if a lymphocyte reacts to a self-antigen → it is eliminated
Clonal Selection of B & T cells
• When a B or T cell interacts with its specific antigen, it is selected and becomes activated
• Activation results in a proliferation, producing a large number of clones
- Each clone is reactive against the antigen that initially stimulated the original lymphocyte Clonal Selection
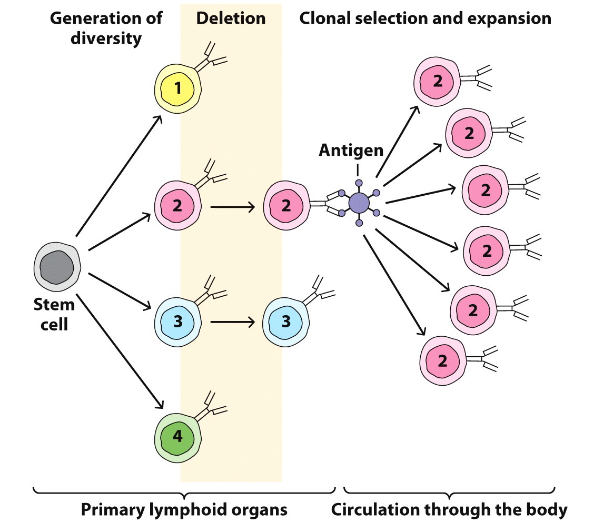
Effector T and B cells
Once T and B cells are activated in lymphoid organs, they become effector cells that can fight infections
This happens through both humoral and cell-mediated activities
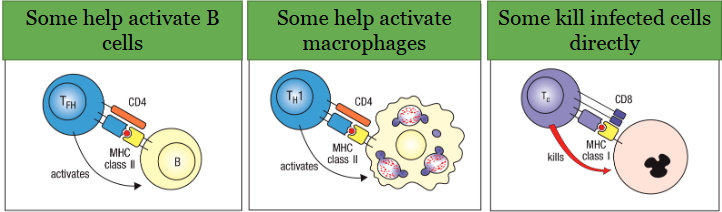
Cell-mediated immunity
Mediated by T cells:
• Contribute to adaptive immunity in many ways
• Many different T cell subsets can get activated depending on the situation and exert a variety of effector functions:
Humoral immunity
Mediated by antibodies produced by B cells:
• Contribute to adaptive immunity by producing specific antibodies
• Antibodies
- There are different types
- Can act in different ways
- Involved in clearing and/or neutralizing antigen
What happens after the immune response? (after the pathogen has been neutralized)
Downregulation of lymphocytes and immunological memory
Natural & induced active adaptive immune response can be achieved by?
• Natural: natural infection
• Induced: vaccination
Natural & induced passive adaptive immune response can be achieved by?
With cells and/or molecules that mediate immunity:
• Natural: mother-to-fetus transfer of antibodies
• Induced: monoclonal antibody therapy
How do pathogens enter the body to cause an infection? (What MUST they pass through?)
Pathogens must break through mucosal and epithelial (ex. skin) surfaces
Different routes of entry
What are the epithelial surfaces of the body?
Epithelial surfaces of the body provide the first barrier against infection:
- Skin
- Gut epithelium
- Respiratory epithelium
- Mucosal membranes
• Saliva, hair, mucus, tears all provide innate immunity
Function of epithelial surfaces of the body? (How do they achieve this function)
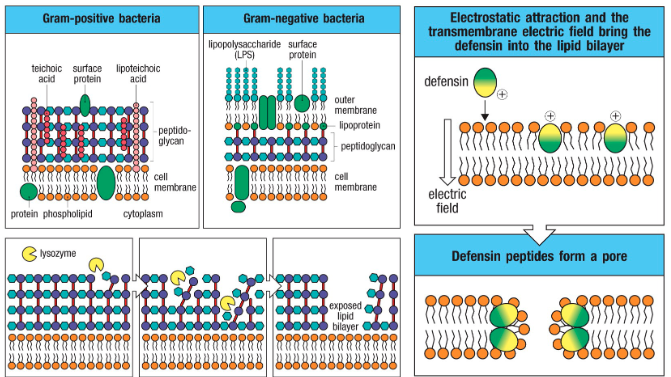
Epithelial layers produce protective substances
- Acidic pH
- Antimicrobial peptides (ex. Defensins)
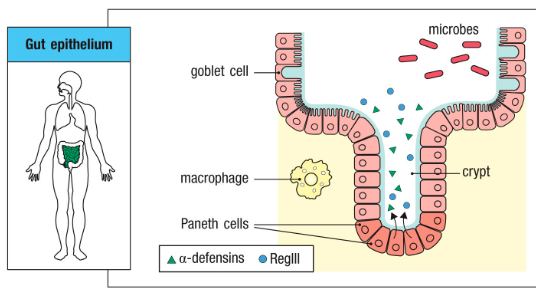
What are the cells that participate in innate immunity?
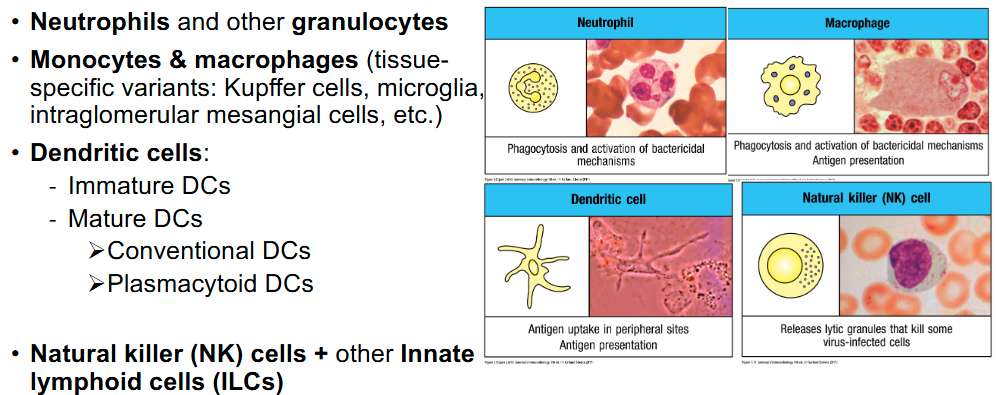
What are the antimicrobial molecules involved in innate immunity?
• Antimicrobial enzymes (eg lysozyme → digest peptidoglycan <cell wall of bacteria>)
• Antimicrobial peptides (eg defensins → disrupt cell membrane)
• Complement
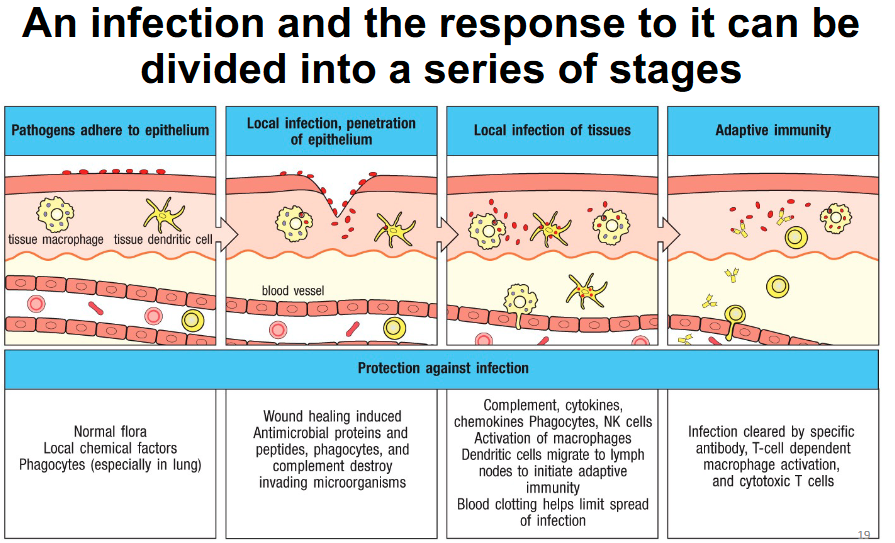
Infection response stages
Phagocytosis
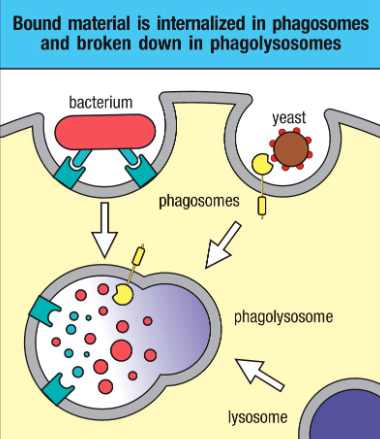
Defined as engulfment and internalization of pathogens or their components upon their binding to receptors on the cell surface of phagocytes:
- Removal and killing of pathogens
- Clearing debris (PRRs recognize DAMPs)
- Generation of peptides for presentation to T cell
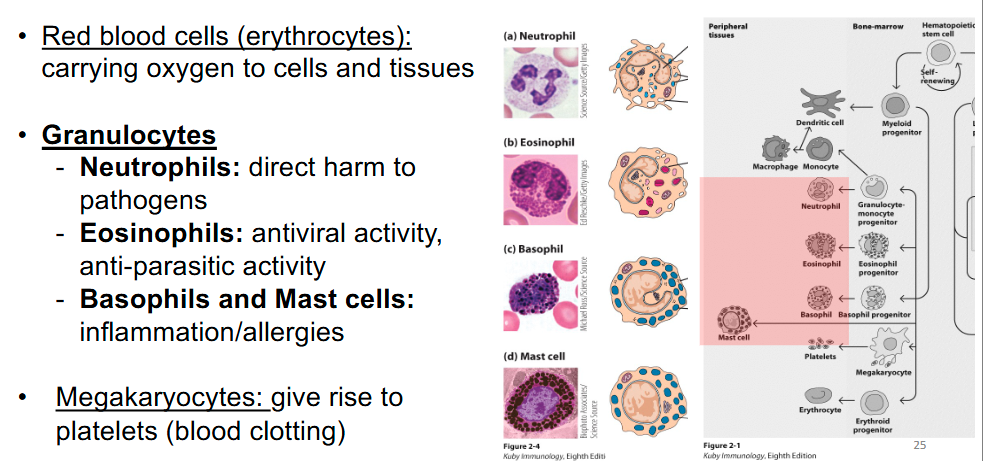
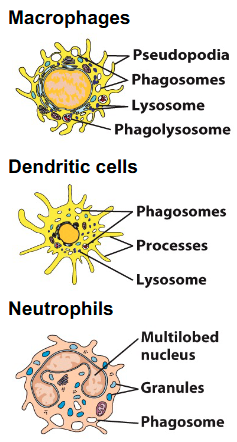
What are the phagocytes?
- Macrophages
- Granulocytes: Neutrophils
- Dendritic cells
Are receptors needed for phagocytosis? If so, what kinds?
Receptors are involved in mediating phagocytosis, many of which are PRRs (Not all PRRs induce phagocytosis)
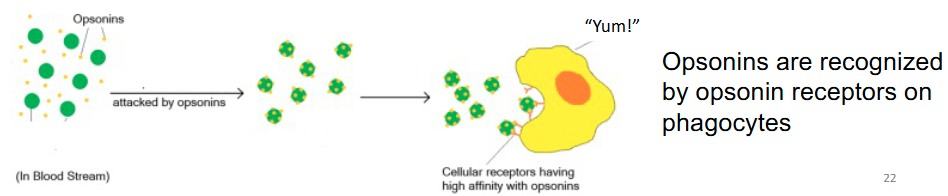
Opsin receptors can indirectly mediate phagocytosis of bacteria
Indirect phagocytosis
Phagocyte recognition of soluble proteins that are bound to microbial surfaces (opsonins), also known as soluble pattern-recognition proteins → enhancing phagocytosis (opsonization) ex. Antibodies and complement proteins
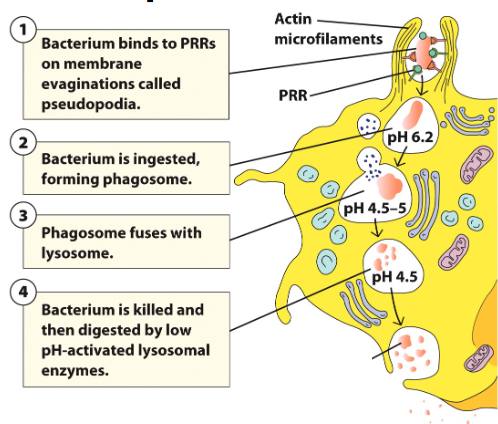
Phagocytosis steps
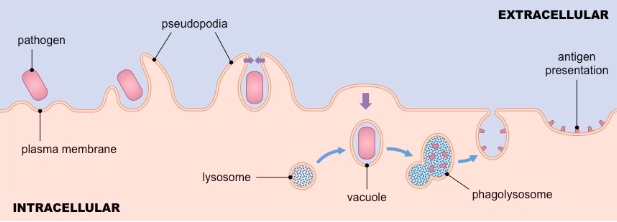
• Phagocytosis initiated when receptors interact with ligand/pathogen
• Prompts membrane protrusions that extend, called pseudopodia
• Pathogen internalized in a large membrane-enclosed endocytic vesicle known as phagosome
• Phagosome fuses with one or more lysosomes → phagolysosome, in which lysosomal content are released
• Phagolysosome acidifies, acquires antimicrobial peptides and enzymes to kill pathogen
How is neutrophil phagocytosis different from other phagocytes?
• Neutrophils contain different types of cytoplasmic granules:
- Primary granules
- Secondary granules
• These granules fuse with phagosomes (phagolysosome), releasing additional enzymes and antimicrobial peptides that attack the microorganism
What kills the phagocytosed pathogen?
Phagolysosomes contain products that can kill microbes
Low pH/ acidification
Hydrolytic enzymes (lysozymes and proteases )
Oxidative attack à employs reactive oxygen species (ROS) and reactive nitrogen species (RNS)
Antimicrobial peptides (eg. defensins and cathelicidin)
What is ROS? How is it generated?
• Employs reactive oxygen species (ROS) → damage microbial membranes and intracellular components
• ROS are generated by phagocytes’ unique NADPH oxidase enzyme complex (or phagosome NADPH oxidase)
• ROS production by NADPH oxidase increases oxygen consumption → respiratory burst
Function of the phagolysosome (macrophage) in innate immunity?
Pathogen killing, Pathogen processing, pathogen presentation to sensory cytosolic PRRs (TLR, NODs)
Function of the phagolysosome (macrophage) in adaptive immunity?
Antigen degradation, antigen processing and antigen presentation onto MHC molecules
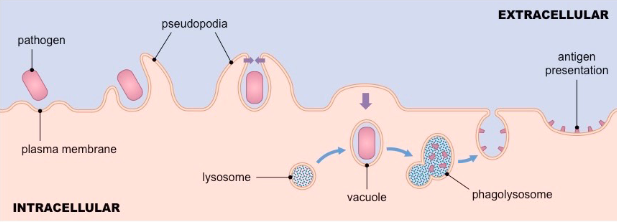
Which phagocyte is non-resident (i.e. always moving around)
Neutrophils
Recruited to site of infection
What is pus?
Pus is result of dead & dying neutrophils
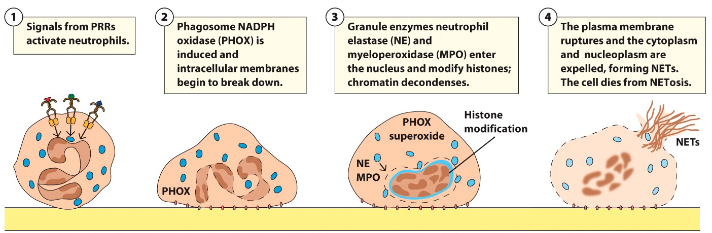
What are NETs?
20-60% of neutrophils can produce extracellular matrix called NETs (neutrophil extracellular traps) → Trap microorganisms and prevent spread
Microglia function?
Homeostasis
• CNS-resident microglia (“macrophages of the brain”) are responsible for establishing proper neuronal connections
– Participate in debris clean-up
– Brain development
– Memory, learning
What is the Complement system?
Term refers to a group of soluble proteins that cooperate with both the innate and adaptive immune systems to eliminate pathogens, dying cells and immune complexes from the body
Proteases (>30) in blood and other fluid
Where are complement proteins made?
The liver
Mechanism of the complement system
Key mechanisms of action:
- Increasing vascular permeability and chemotaxis (inflammation)
- Destroying pathogen cell membranes
- Increasing recognition of pathogens and facilitating phagocytosis
(opsonization)
Opsonization
“the coating of the surface of a pathogen by antibody and/or complement (soluble proteins) that makes it more easily ingested by phagocytes”
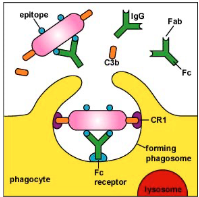
Do neutrophils have lysosomes?
No, they have granules
Phagosome + granules are still called a phagolysosome
What is the inactive form of the complement system?
Pro-proteases
What are the 3 ways to activate the complement system?
- Classical pathway
- Alternative pathway
- Lectin pathway
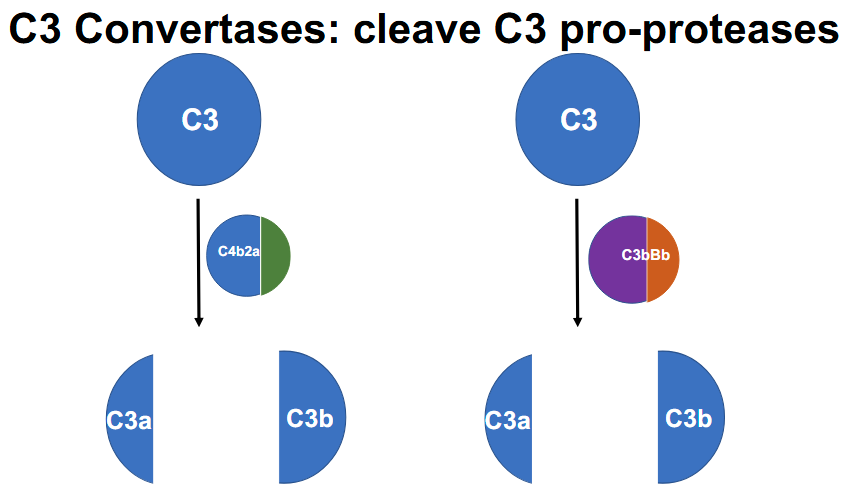
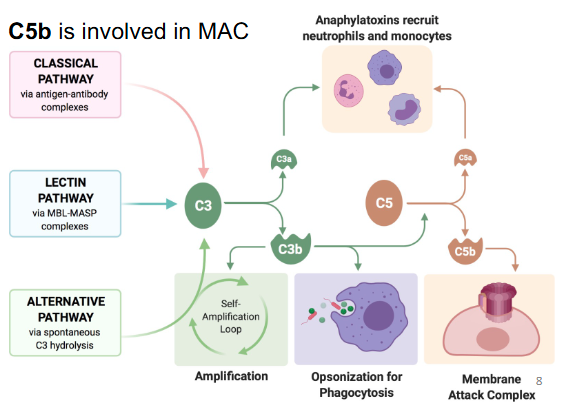
All three pathways generate C3 convertase (which cleaves C3 → C3a + C3b)
Explain the steps of complement activation
Proteolytic cleavage generating two fragments:
• One small:
- Identified by the letter “a” after the name (e.g. C5a)
- With a specific function
• One large:
- Identified by the letter “b” after the name (e.g. C5b)
- With proteolytic activity on a new substrate
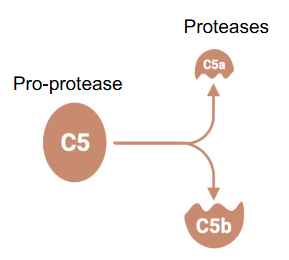
After cleavage which of the 2 protease fragments has proteolytic activity?
The large one
Identified by the letter “b” after the name (e.g. C5b)
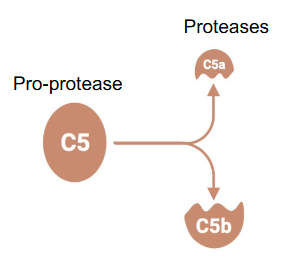
Function of the complement system?
Opsonization
Increased inflammation
Membrane attack complex
What are the 2 C3 (pro-protease) convertases (cleaves C3)
1.) C4B2A
2.) C3bBb
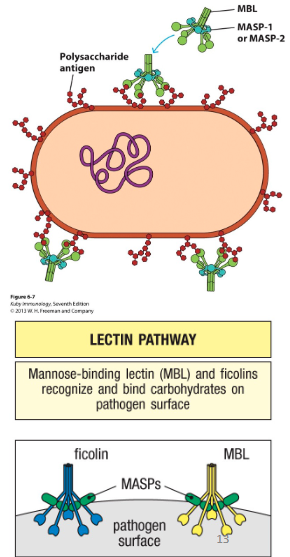
What are lectins?
PRRs that circulate in the blood
Activates the Lectin pathway
- Mannose-binding lectin (or MBL)
- Ficolins
Lectin Pathway
• Expression of lectins increase during infection
• These PRRs can bind surface of pathogens
- This activates MASPs (MBL-associated serine proteases), which triggers signaling cascade on pathogen surface
- C3 convertase is generated (C4b2a)
- C3 cleaved → C3a and C3b
Classical Pathway
C1q binds pathogen surface
- Can bind pathogen directly
- Can bind antibodies that are bound to pathogen surface
**This can connect adaptive to innate**
Once C1q binds
- This changes the confirmation of the two serine
proteases (C1r and C1s), which triggers signaling
cascade on pathogen surface
- C3 convertase is generated (C4b2a)
C3 cleaved → C3a and C3b
What is the end result of both the classical and lectin pathways?
• Classical and lectin pathways result in generation of C3 convertase (C4b2a)
• C3 convertase CLEAVES C3 → C3a and C3b
C3a and C3b function?
• C3a: Involved in enhancing inflammation
• C3b: Involved in Opsonization, and is a C5 convertase à C5a and C5b
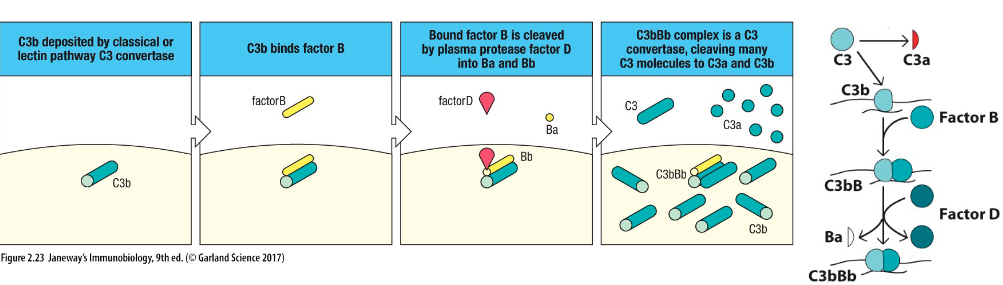
How is C3bBb formed?
Alternative pathway:
Factor B binds to C3b
Factor D cleaves Factor B into Ba and Bb
Results in C3bBb
How is the C3(H2O)Bb complex formed? What is it’s function?
Alternative pathway:
High concentration of C3 undergoes hydrolysis
Factor B binds and Factor D cleaves it into Ba and Bb
Results in C3(H2O)Bb
The complex is a C3 convertase and produces C3a and C3b
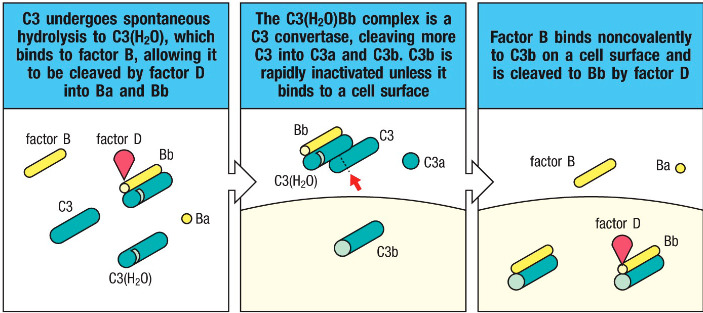
Is C3bBb stable? If not, what stabilizes it?
• The alternative pathway C3 convertase (C3bBb) are
very unstable
- Stabilized by factor called properdin (factor P)
secreted by neutrophils
- Properdin can stabilize C3 convertase since it can
bind to some microbial surfaces
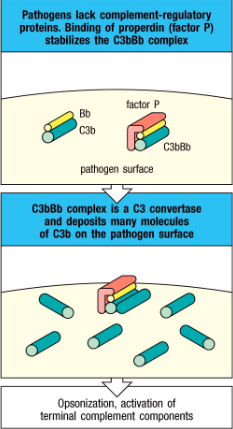
Properdin function?
Stabilizes C3b
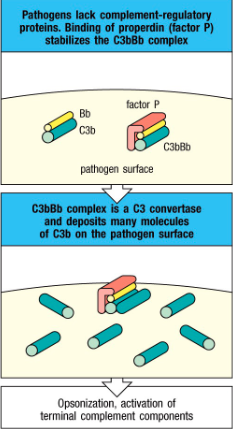
Extracellular bacteria:
Complement occurs outside the cell surface
How is inflammation enhanced by the complement system?
Additional signaling results in cleavage of other complement molecules
C3a and C5a recruit phagocytes and promote inflammation by vasodilatation
If present in large amounts, C3a and
C5a → anaphylactic shock
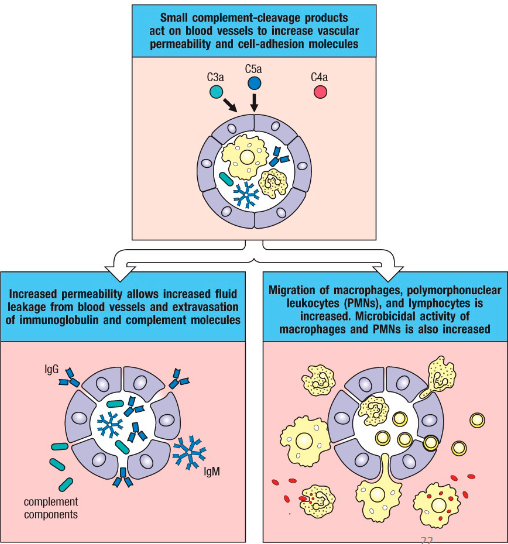
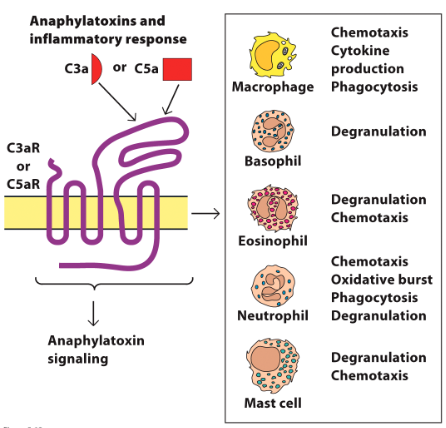
C3a and C5a mechanism of action in promoting inflammation
Binds to C3aR/C5aR on granulocytes:
Stimulates release of proinflammatory cytokines and granule components from basophils, eosinophils, neutrophils, mast cells
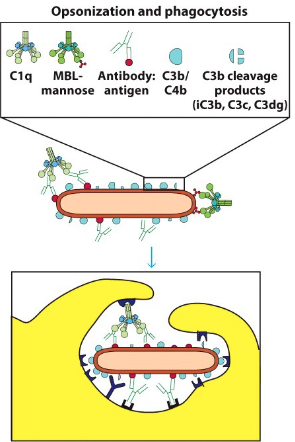
How does C3b enhance phagocytosis?
- Phagocytes have receptors for C3b
- Opsonization of pathogen → more readily taken up by phagocytosis (i.e. C3b binds to pathogen then phagocyte)
- Note: opsonization can occur via complement deposition and/or antibodies (phagocytes also have receptors for antibodies)
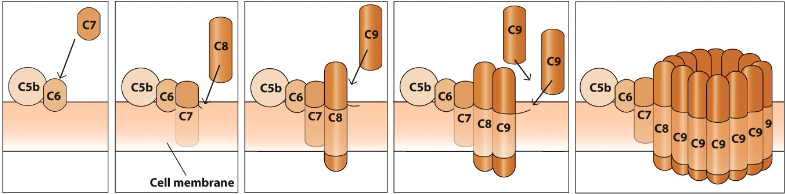
Membrane attack complex
- Additional complement factors create membrane-attack complex
(MAC) → Cell lysis!
- C5 and C3 are involved (C5b directly involved and C3b indirectly)
- C3b is indirectly involved as it is a C5 convertase
Regulation of C activation
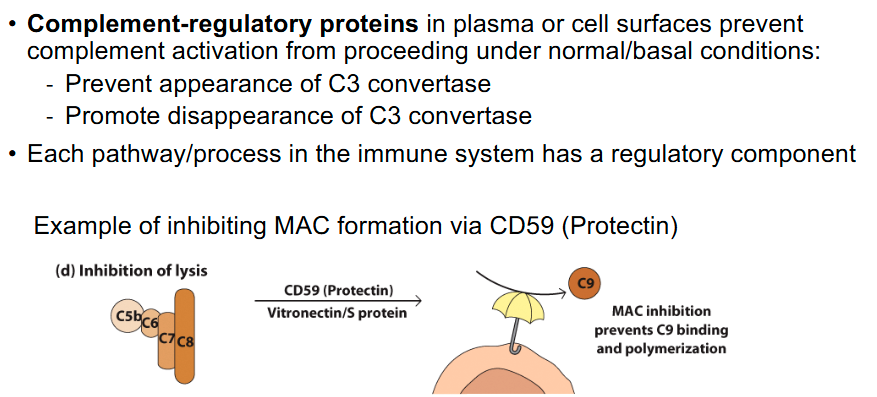
Functions of C3, C3b, C3a, C5a, and C5b?
• C3 convertase (C4b2a) and (C3bBb) → C3a and C3b
• C3b involved in opsonization, is a C5 convertase (indirectly involved in MAC)
• C3a involved in inflammation
• C5a involved in inflammation
• C5b is involved in MAC
Pathogen-Associated Molecular Patterns (PAMPS)
Pathogens have specific molecular patterns that support their lifestyle
What do PRRs recognize?
• PAMPs are recognized by Pattern Recognition Receptors (PRRs), which are on host cells, in host cells and are host soluble proteins
– This range of locations ensure that cells can recognize the PAMPs of virtually any pathogen
• Damage-Associated Molecular Patterns (DAMPs) are also recognized by PRRs
Which cells express PRRs?
• All types of myeloid white blood cells
• Subset of the lymphoid cells: T cells, B cells, NK cells
• PRRs are also expressed by some other cell types
- Those commonly exposed to infectious agents (eg. Epithelial cells of the skin and mucosal tissues and endothelial cells that line the blood vessels, leading to production of antimicrobial substances
What are cytosolic sensors? What cells express them?
Cytosolic sensors of viral nucleic acids are expressed by most if not all cells in the body
Location of PRRs?
Located at different sites depending on the PAMP they recognize:
– Cell surface (for bacteria)
– Intracellular (for virus)
– Secreted
Third answer (needs factor D also)
What are DAMPS?
Signals cell release when they are dying.
What are the different groups of PRRs?
Several groups:
– Toll-like receptors (TLRs)
– NOD-like receptors (NLRs)
– RIG-I-like-receptors (RLRs)
– C-type lectin receptors (CLRs)
– Ficolins, MBL, C1q
– Others
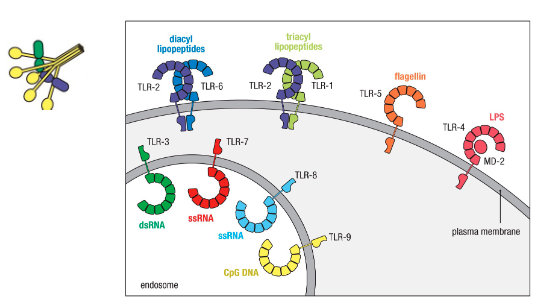
Toll-like receptors locations?
Intracellular vs extracellular receptors → logic behind corresponding PAMPs (location helps determine what each binds):
Intracellular for viruses
Extracellular for bacteria
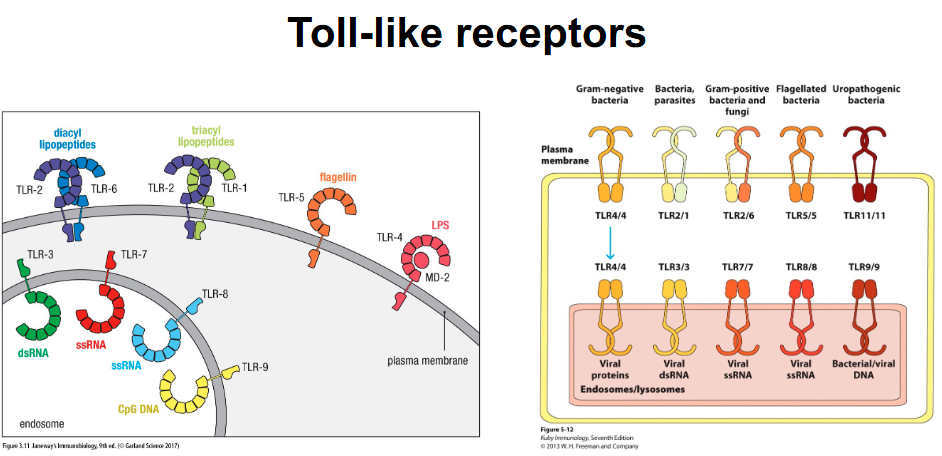
What happens after TLRs bind to PAMPs?
Different TLRs recruit different adaptor proteins – link protein-binding partners together and facilitate large signaling complexes
• Different adaptor proteins lead to different events that include:
– NF-κB transcription factor activation
– Interferon regulating factor (IRF) pathways
– MAP kinase pathway downstream transcription factors (AP-1)
• NF-κB, IRF and AP-1 are transcription factors
• Phosphorylation is a key event for activation

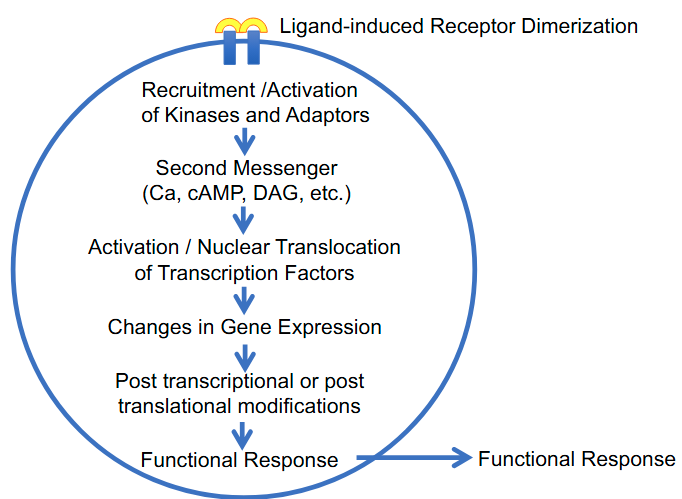
General Features of Signal Transduction