Neurotransmitters System II: GABA and Glycine
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What is GABA and where in the CNS is it most commonly used?
GABA (γ-aminobutyric acid) is the major inhibitory neurotransmitter in the adult CNS.
Inhibits AP
~1/3 of all synapses use GABA.
Predominantly used by local circuit interneurons (short-range inhibitory neurons that shape network activity).
GABAergic signalling is essential for controlling excitability, preventing runaway excitation, synchronising rhythms, and shaping outputs like movement (e.g., cerebellar Purkinje cells).
How is GABA synthesised?
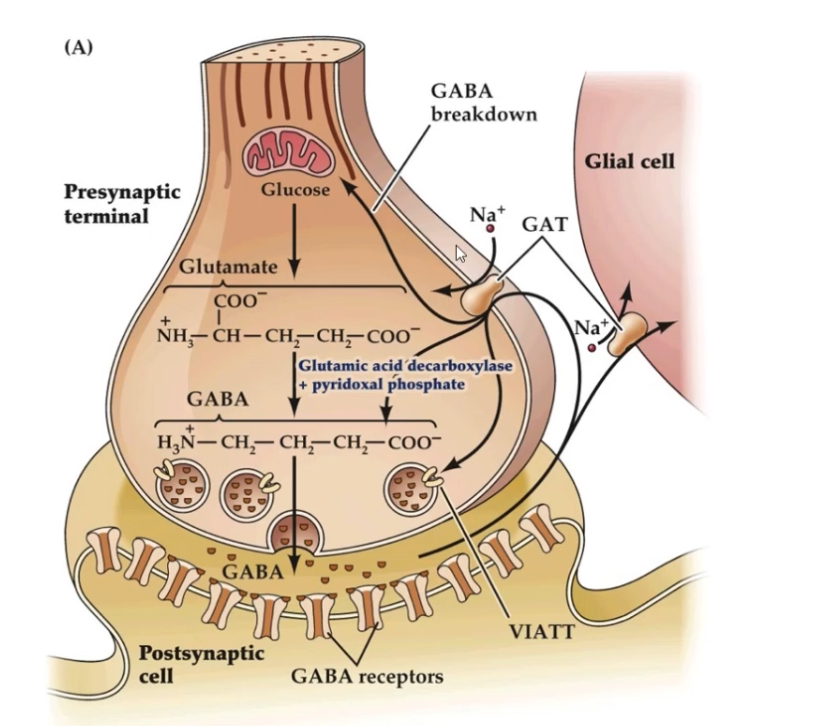
Glutamate —> GABA via, GAD and PLP
Precursor: Glutamate.
Enzyme: Glutamate decarboxylase
Cofactor: PLP (pyridoxal phosphate), derived from vitamin B6 – crucial for catalytic activity.
Location: Synthesised in nerve terminals.
How is GABA stored in synaptic terminals?
Loaded into vesicles by the Vesicular Inhibitory Amino Acid Transporter (VIAAT / VGAT).
Stored in oval-shaped vesicles (distinct from round glutamate vesicles).
VIAAT transports both GABA and glycine, explaining why some inhibitory terminals co-release them.
GABA reputable and degradation
GABA signalling is terminated either by re-uptake into cells or by enzymatic degradation.
Re-uptake:
GABA is released into the synaptic cleft from the presynaptic neuron
After binding to GABA receptors, excess GABA must be cleared to stop inhibition
GABA transporters (GATs) move GABA back into cells using a Na⁺-dependent mechanism
Neurons mainly use GAT-1
Glial cells (astrocytes) mainly use GAT-3
Re-uptake:
Terminates the inhibitory signal
Recycles GABA for future use
Prevents prolonged inhibition
Degradation:
Once inside the cell (especially astrocytes), GABA can be broken down
GABA → succinic semialdehyde
Enzyme: GABA transaminase (GABA-T)
Succinic semialdehyde → succinic acid
Enzyme: succinic semialdehyde dehydrogenase (SSADH)
Succinic acid enters the TCA (Krebs) cycle
This links neurotransmitter metabolism to cellular energy production
Why this matters:
Tight control of GABA levels prevents excessive inhibition
Drugs that inhibit GABA-T (e.g. vigabatrin) increase GABA levels and enhance inhibition
What are the two receptor families GABA acts on?
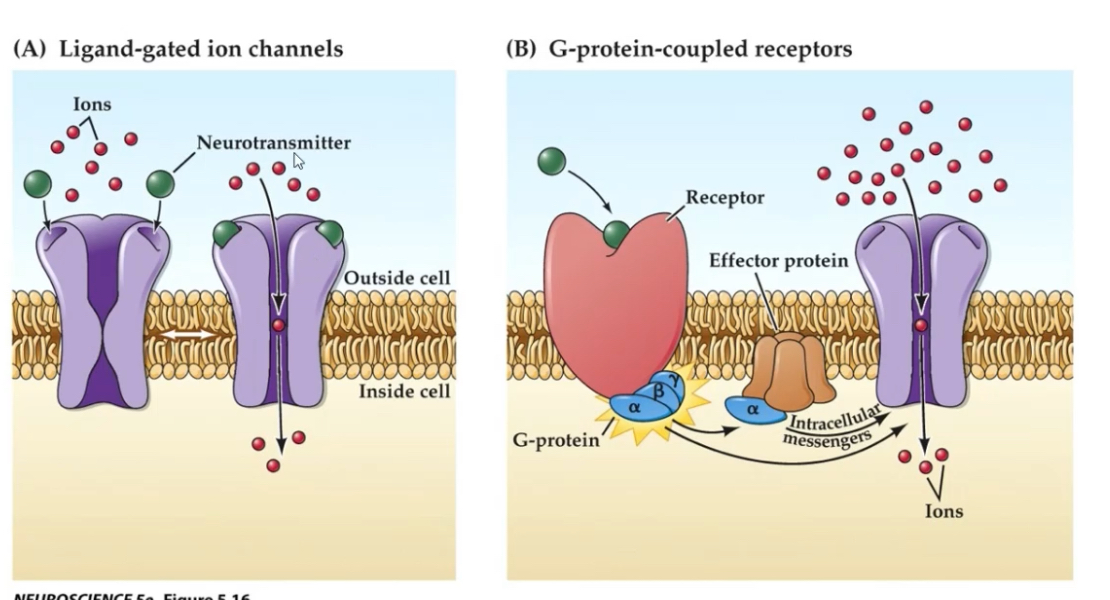
1. GABA_A — Ionotropic, ligand-gated Cl⁻ channel.
2. GABA_B — Metabotropic, GPCR, Gi/o-coupled.
Describe the structure of the GABA_A receptor.
GABA_A receptors inhibit neurons by opening Cl⁻ channels, hyperpolarising the postsynaptic membrane and reducing firing.
Ligand-gated Cl⁻ channel:
Receptor is closed without ligand
GABA binds to the receptor
Channel opens
Cl⁻ ions flow into the neuron
Membrane potential becomes more negative (hyperpolarisation)
Neuron is less likely to fire an action potential
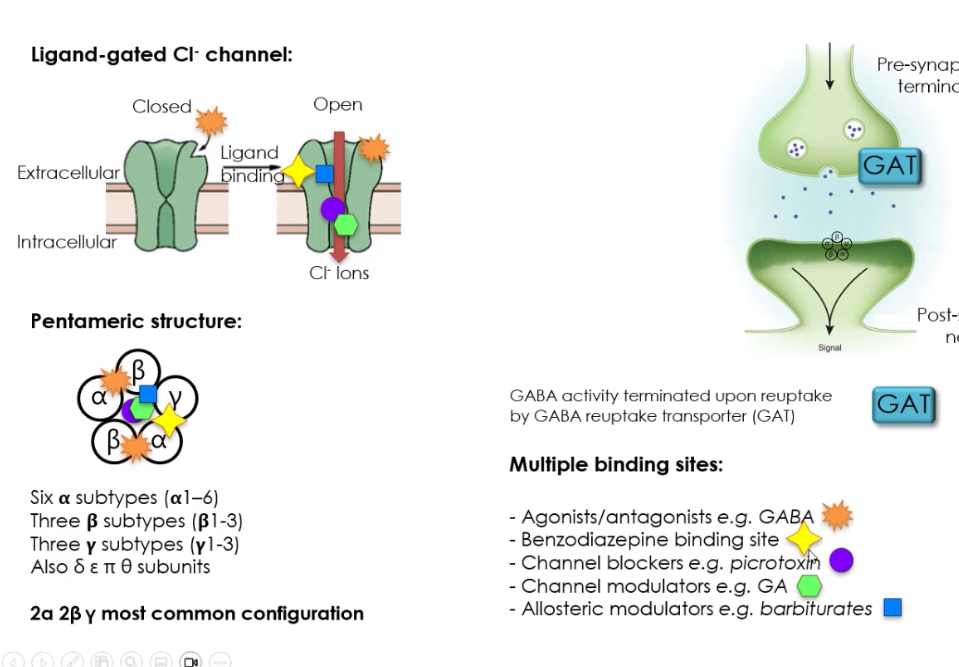
Receptor structure:
Pentameric receptor → made of 5 subunits
Many possible subunits:
α (1–6)
β (1–3)
γ (1–3)
Also δ, ε, θ
Most common configuration: 2α + 2β + 1γ
Subunit composition affects:
Drug sensitivity
Kinetics
Brain location
Binding sites on GABA_A receptor :
GABA site
Agonists activate the receptor
Benzodiazepine site
Enhances GABA effect (increases Cl⁻ channel opening frequency)
Channel blockers (e.g. picrotoxin)
Physically block Cl⁻ flow
Channel modulators
Alter how long or how often the channel opens
Allosteric modulators (e.g. barbiturates)
Increase inhibitory effect without activating receptor alone
Termination of GABA signal:
GABA removed from synaptic cleft by GABA transporters (GAT)
Ends inhibitory signalling
Allows precise control of inhibition
Describe the signalling pathway of the GABA_B receptor.
GPCR composed of GABA_B1 + GABA_B2 heterodimer.
Couples to Gi/o →
Inhibits adenylyl cyclase → ↓cAMP
Opens K⁺ channels so it flows out of post synaptic neuron so it’s more negative → hyperpolarisation
Inhibits Ca²⁺ channels from entering→ reduces neurotransmitter release
Presynaptic GABA_B = reduces transmitter release (glutamate or GABA).
Postsynaptic GABA_B = slow inhibitory postsynaptic potentials (IPSPs).
Why is GABA relevant to epilepsy treatment?
Epilepsy involves excessive synchronous neuronal firing.
Increasing GABAergic inhibition helps reduce excitability.
Drugs: benzodiazepines, barbiturates, vigabatrin (GABA-T inhibitor), tiagabine (GAT inhibitor).
The Cerebellum and GABA
Purkinje cells slow things down to fine-tune movement.
Purkinje cells are GABAergic (inhibitory) neurons in the cerebellum
They receive lots of input on their large dendrites
They send inhibitory signals to the deep cerebellar nuclei
This inhibition acts as an error signal
It helps adjust and smooth movements in real time
Each Purkinje cell receives ~200,000 synapses, making them among the most heavily integrated neurons.
Glycine
Glycine is the second major inhibitory neurotransmitter in the CNS.
Most prominent in ventral horn of spinal cord and brainstem.
Glycinergic circuits are essential for motor reflexes, posture control, and respiratory rhythm generation.
How is glycine synthesised?
Precursor: Serine.
Enzyme: Serine hydroxymethyltransferase (SHMT).
Occurs in nerve terminals.
SHMT links glycine synthesis to one-carbon metabolism (folate cycle).
Degradation uses the same enzyme(SHMT) to turn glycine back to serine
How is glycine stored?
Packed into vesicles via VIAAT / VGAT, same transporter as GABA.
Vesicles are oval in inhibitory terminals.
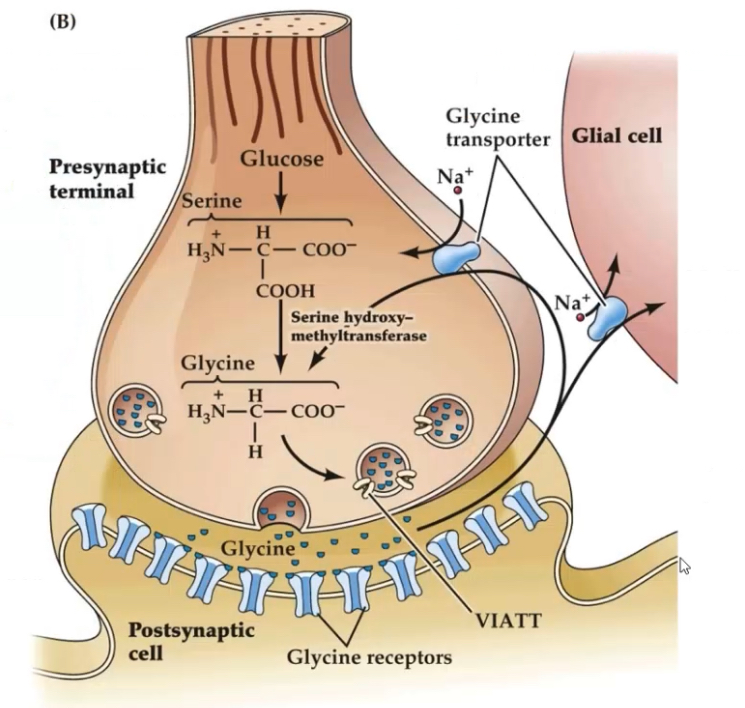
How is glycine removed from the synaptic cleft?
GlyT-1 (glial transporters).
GlyT-2 (neuronal transporters).
GlyT-2 mutations cause hyperekplexia (startle disease) because glycinergic inhibition fails → hyperexcitability.
Describe the structure of glycine receptors.
Ligand gated
Pentameric Cl⁻ channels.
Subunits:
α1–α4
β
Most common: 3α1 + 2β, or 4α1 + 1β.
What blocks Glycine receptors?
The plant alkaloid strychnine is a potent antagonist.
Strychnine poisoning produces severe muscle spasms and can cause death due to respiratory failure (loss of spinal inhibition).
Cl⁻ influx through the ion channel → neuronal hyperpolarisation → inhibition of action potential firing.
Signalling terminated by GlyT-1/GlyT-2 reuptake.
How do glycine receptor mutations cause hyperekplexia?
Mutations in GlyR α1/β or GlyT-2 reduce Cl⁻ conductance.
Leads to reduced inhibitory tone → neuronal hyperexcitability → exaggerated startle response and muscle rigidity.
What key features do GABA and glycine share?
Both inhibitory neurotransmitters.
Both use VIAAT/VGAT for vesicular loading.
Both act on Cl⁻ permeable ligand-gated ion channels (GABA_A and GlyR).
Both terminated mainly by reuptake transporters (GATs vs GlyTs).
Both crucial for motor coordination, inhibition, and preventing hyperexcitability.
What are major differences between GABA and glycine?
Location:
GABA = brain-wide; interneurons, cerebellum
Glycine = spinal cord, brainstem
Receptor diversity:
GABA has both ionotropic (A) and metabotropic (B) receptors.
Glycine has only ionotropic receptors.
Pharmacology:
GABA_A allosteric modulators are numerous (benzos, barbiturates).
GlyR modulators are limited; strychnine is main antagonist.
Clinical relevance:
GABA: anxiety, epilepsy, sedation.
Glycine: hyperekplexia, motor reflexes.