10. Branches Alkanes + degree carbons + constitutional isomers!
0.0(0)
Card Sorting
1/7
Earn XP
Description and Tags
page 11 in packet
Last updated 3:50 AM on 3/24/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
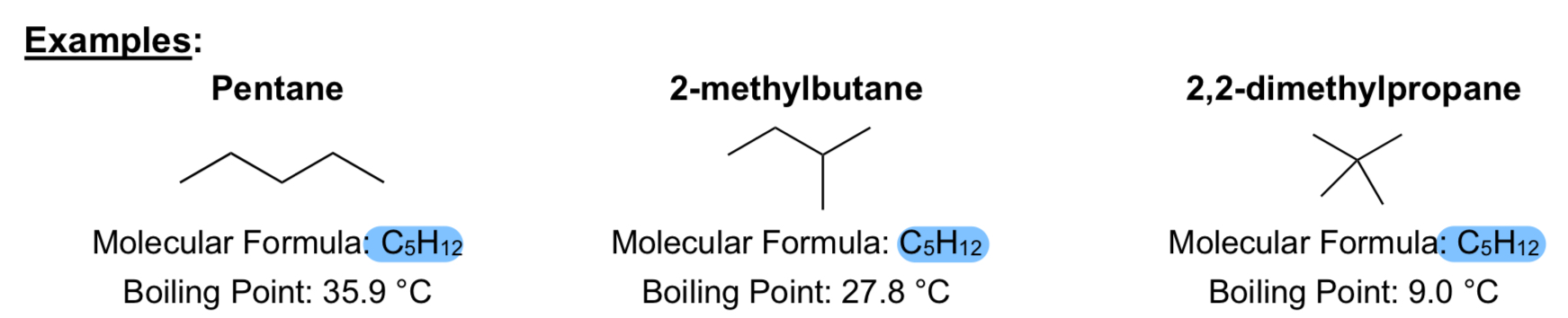
What are n-alkanes?
Alkanes with an unbranched carbon chain
2
New cards
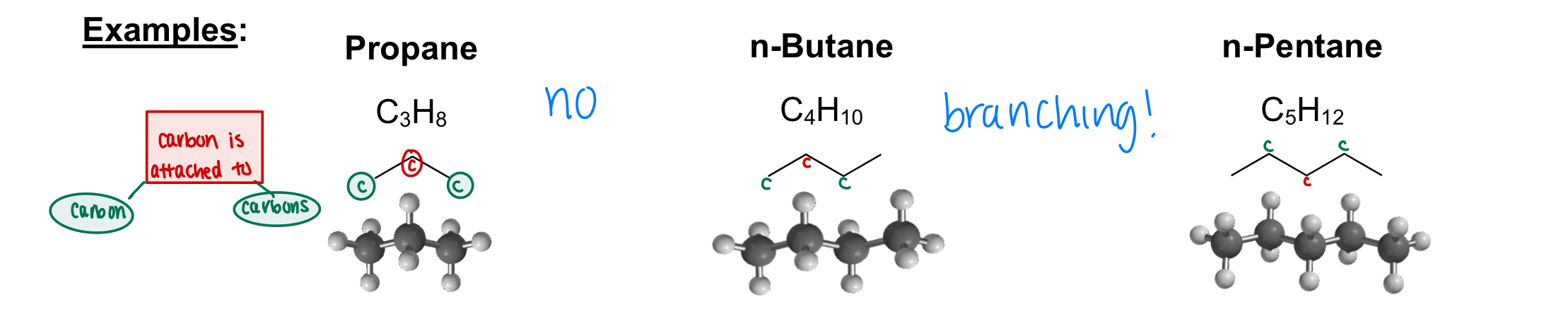
What are branches alkanes?
Alkanes whose carbon atoms can bind up to 4 other carbon atoms
3
New cards
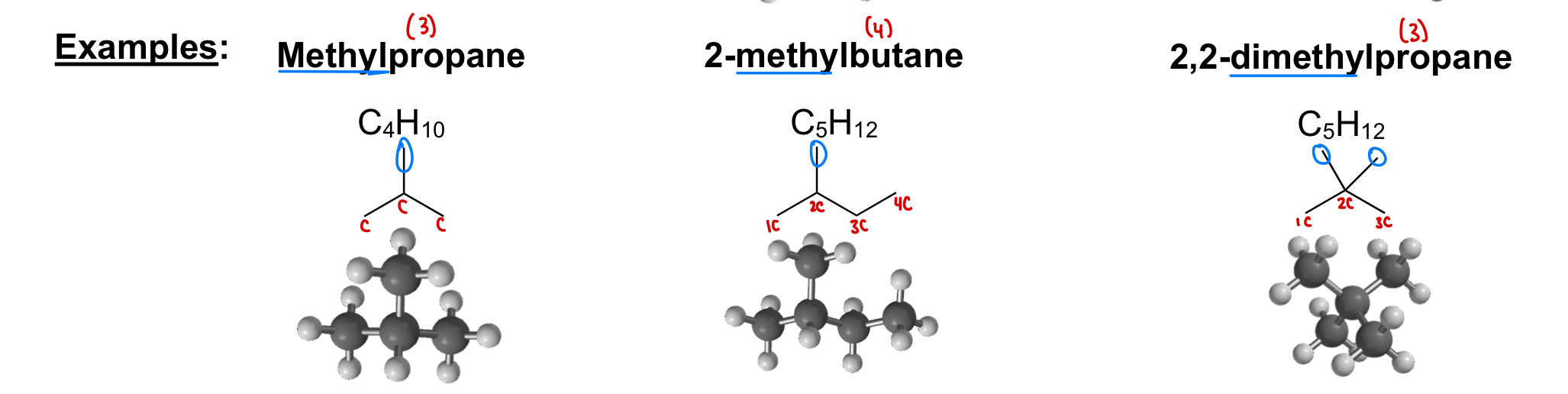
What is a methyl carbon (CH3)?
A carbon bonded no other carbons
4
New cards
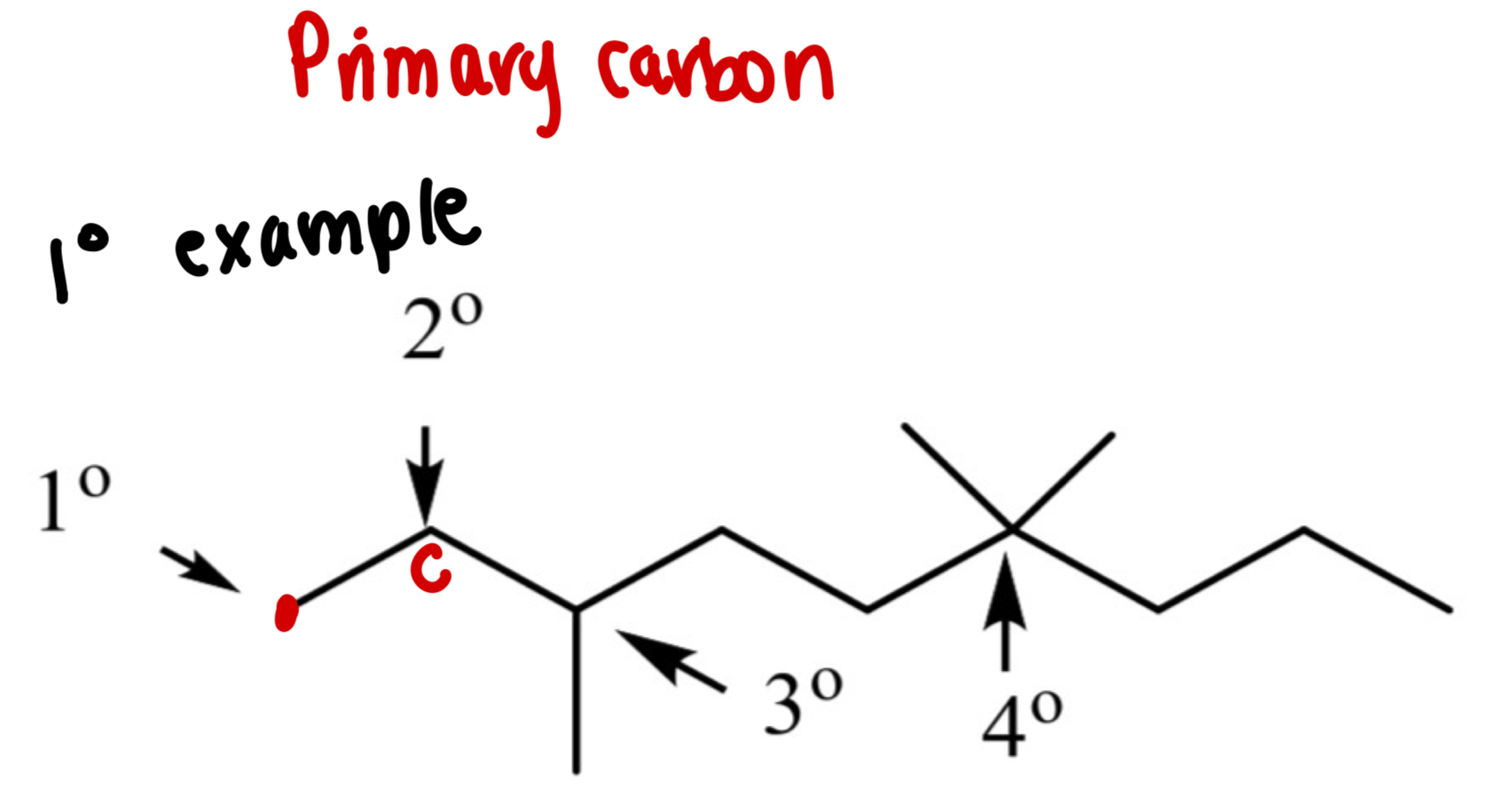
What is a primary carbon (1°)?
A carbon connected to 1 other carbon
5
New cards
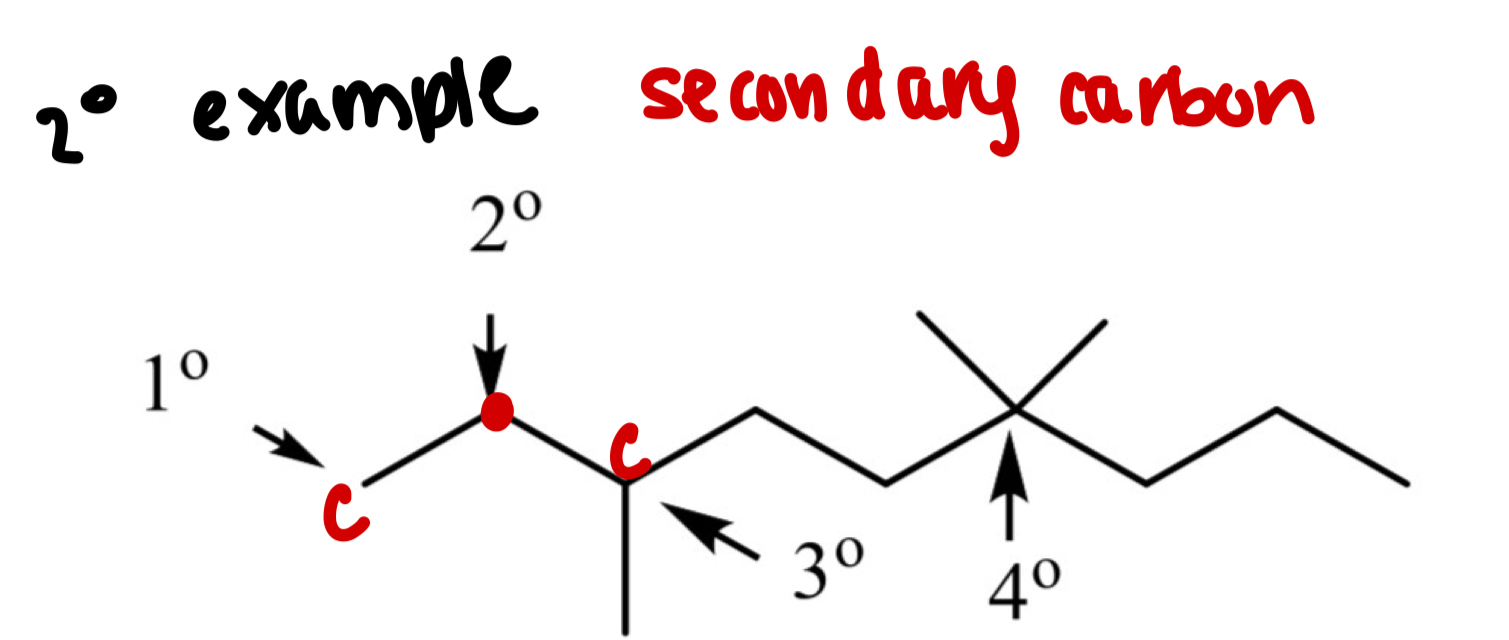
What is a secondary carbon (2°)?
A carbon bonded to 2 other carbons
6
New cards
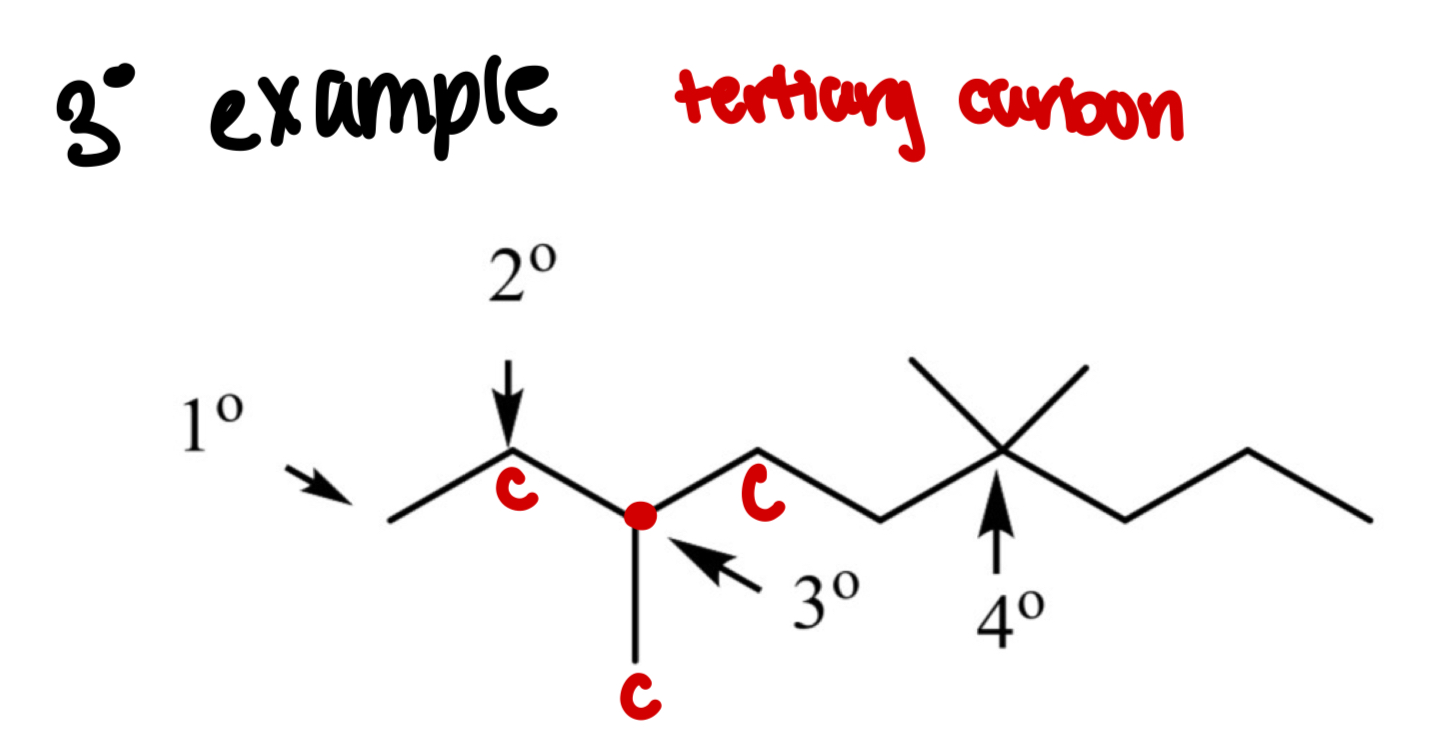
What is a tertiary carbon (3°)?
A carbon bonded to 3 other carbons
7
New cards
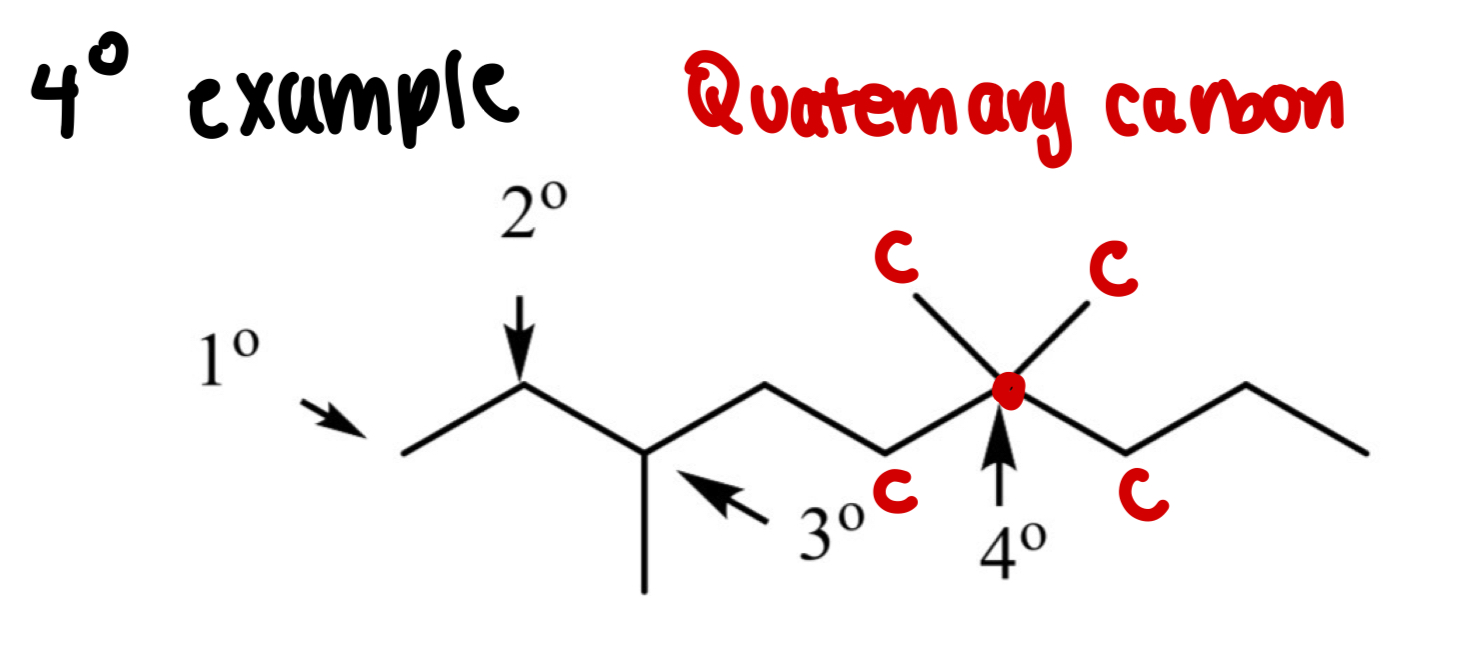
What is a Quaternary carbon (4°)?
A carbon bonded to 4 other carbons
8
New cards
What is a constitutional isomer?
Compounds that have the same molecular formula, but the atoms are connected differently, resulting in different physical and chemical properties