Chemistry
6.1 - Matter and Atoms
Matter
a substance that has volume (occupies space) and has a mass
all matter are made up of atoms which bond together to produce different substances
Atoms
“building blocks of matter” (classic definition of atoms)
1802- first atomic theory of matter presented by john dalton
proposed that all matter is made up of tiny spherical particles which are invisible and indestructible
now know it is incorrect
atoms are made of smaller subatomic particles (protons, neutrons, electrons)
6.2 - The Atomic World
Definitions
Elements: Cannot be broken down into smaller parts by physical or chemical means
Compounds: Made up by two or more elements, can be separated chemically
Molecules: The smallest part of a substance
Compound vs Element: Inseparable vs Separable
Are all elements molecules?: No
Pure substances vs mixtures
matter can be split into two groups
pure substances
mixtures
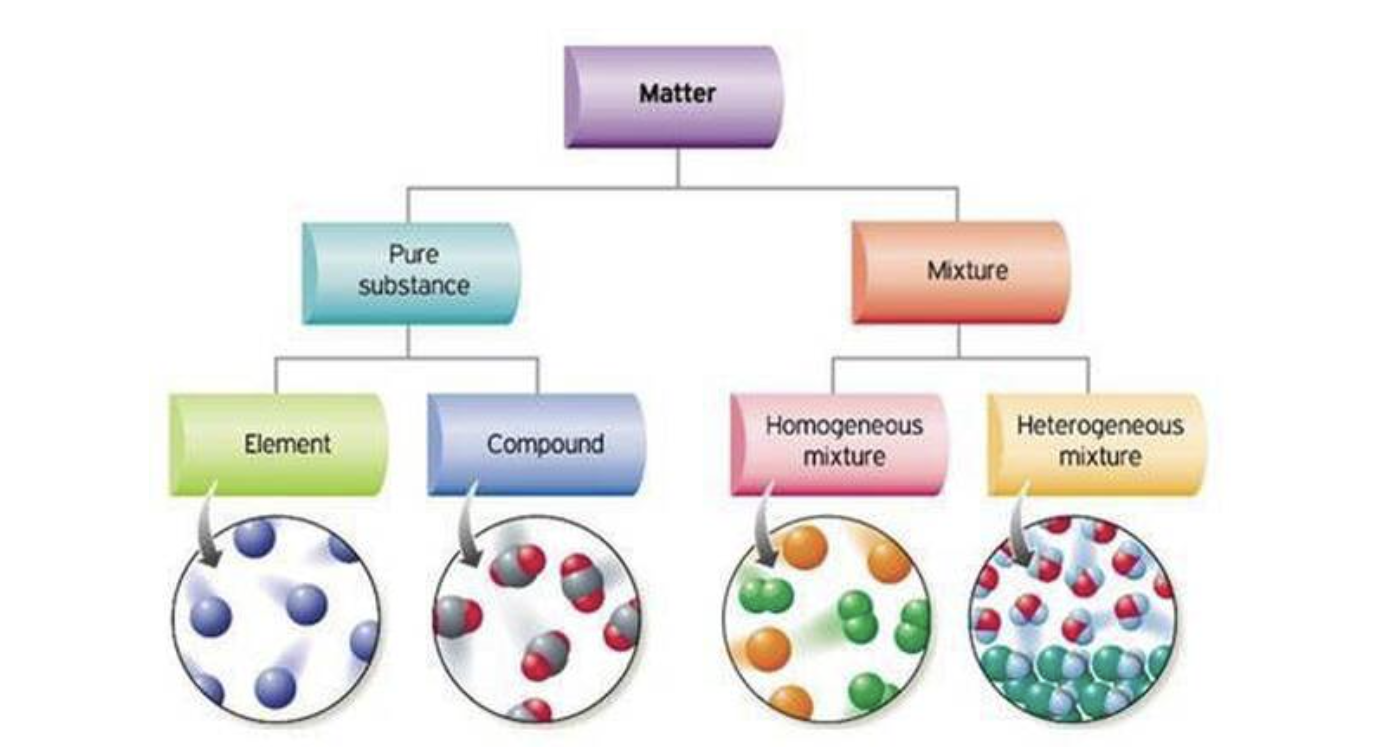
Mixtures
consists of two or more types of particles that are not chemically combined together
can be made elements, compounds or both
e.g. oxygen gas (O2) carbon dioxide (CO2) nitrogen gas (N2)
e.g. oil-water mixture
mixtures can be separated by physical means (e.g. filtration)
Pure Substances
a substance made up of only one type of particle throughout
fixed composition
constant properties
can either be one single element or one single compound
every sample of substance must contain exactly the same thing
must have a fixed, definite set of properties
pure element - copper metal (Cu)
pure compound - (CO2)
cannot be separated by physical means (e.g. filtration)
Elements
made up of just one type of atom
could be monoatomic (exist as individual atoms)
cannot be separated into simpler substances by physical or chemical means
each element has a unique name and chemical symbol
Compounds
different types of atoms combine to form new substances
molecules - two or more atoms that are held together by chemical bonds
Pure Substances: Element or Compound?
to determine whether a pure substance is an element or a compound, you must determine if the substance can be broken down into simpler substances
substance | element or compound |
copper | element |
sulfur | element |
water | compound |
carbon dioxide | compound |
diamond | element |
sodium chloride | compound |
gold | element |
How to Identify if Substances are Pure or Mixture
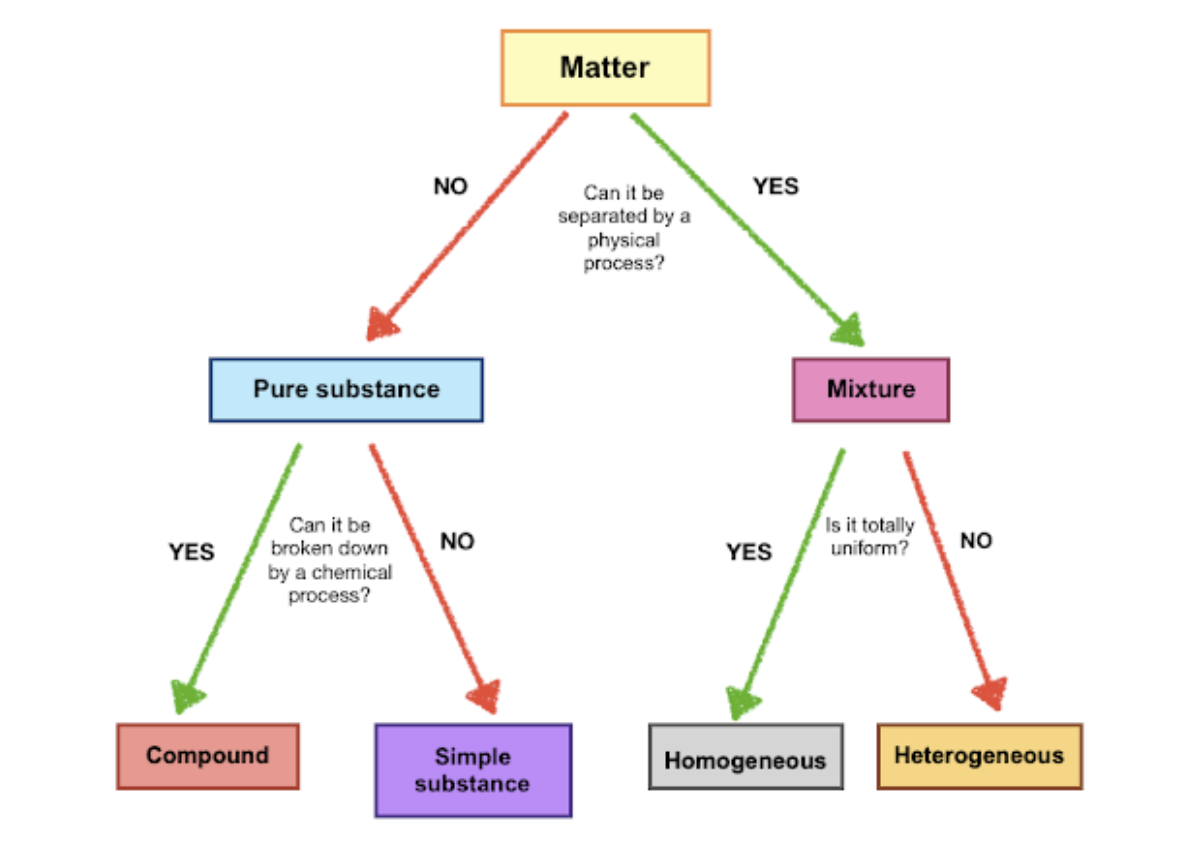
Summary
elements: cannot be broken down into smaller parts by physical or chemical processes (only one type of atom)
molecules: two or more atoms that are held together by chemical bonds (which still holds all its properties), smallest part of a substance
compounds: different types of atoms combined to form a new substance, can be separated chemically
atoms: the smallest part of a substance that cannot be broken down chemically
mixtures: two or more types of particles which are not chemically bound together
pure substances: substances made up of only one type of particle throughout
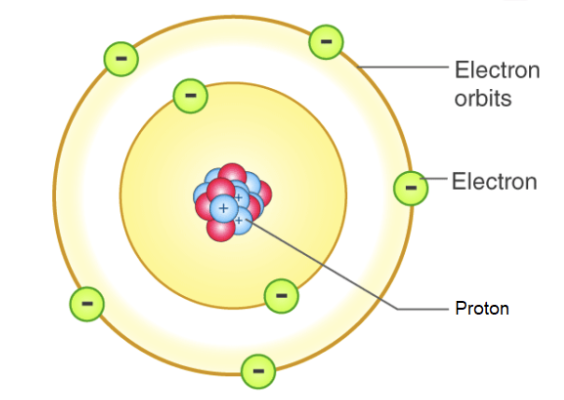
the atomic structure (bohr model):
Element VS Compound
Element
one type of atom
generally monatomic (only one atom)
can be di- or poly- atomic, so long as they are the same type of atoms
e.g. O, C, H (mono)
e.g. H2, O2, N2, C60, (di)
Compound
two or more atoms chemically bonded (e.g. covalent, ionic bonding)
these atoms must be different elements
e.g. O2, H2O, NaCl
Molecule
two or more atoms that are covalently bonded
all molecules are compounds but not all compounds are molecules
(insert venn diagram)
school of thought 1: any atoms chemically bonded are compounds, any covalently bonded compound is a molecule
criteria for being a molecule
made up of atoms that are covalently bonded
made up of atoms of the same or different elements
criteria for both a molecule and compound
made up of atoms that are covalently bonded (makes a molecule)
the atoms are made up of two or more different elements (makes a compound)
school of thought 2: any two different chemically bonded atoms are compounds, any covalently bonded same atoms are molecules
6.3 - Atomic Structure
Atomic Structure
an atom is made up of three sub-atomic particles
protons (positively charged)
neutrons (neutral charge)
electrons (negatively charged)
note: in chemistry, the word particle is a general term that refers to a small unit of matter. depending on the context, ‘particle’ could mean an atom, a molecule, an ion, or something else
protons and neutrons are relatively equal mass and are huge in comparison to electrons. this is why the nucleus is dense
protons and neutrons contribute nearly all the mass of the atom
Rutherford’s Model
most of the mass of an atom, and all of the positive charge must be located in a tiny central region called the nucleus
most of the volume of an electron is empty space, occupied only by electrons
the electrons move in circular orbits around the nucleus
the force of the attraction between the positive nucleus and the negative electrons is electrostatic
prior to rutherford’s experiment, the atom was thought to be a spherical cloud filled with electrons all over (think raisin cakes)
in 1911, rutherford’s experiment proved that the atom had a tiny but heavy nucleus and that most of the volume of an atom is empty space occupied by electrons
The Bohr Model
in 1913, niels bohr developed a new model of the hydrogen atom that explained emission spectra
the bohr model proposed the following:
electrons revolve around the nucleus in fixed, circular orbits
the electrons’ orbits correspond to specific energy levels in the atom
electrons can only occupy fixed energy levels and cannot exist between two energy levels
scientists quickly extended bohr’s model of the hydrogen atom to other atoms
they proposed that electrons were grouped in different energy levels called electron shells. these electron shells are labelled with the number n = 1, 2, 3… 7
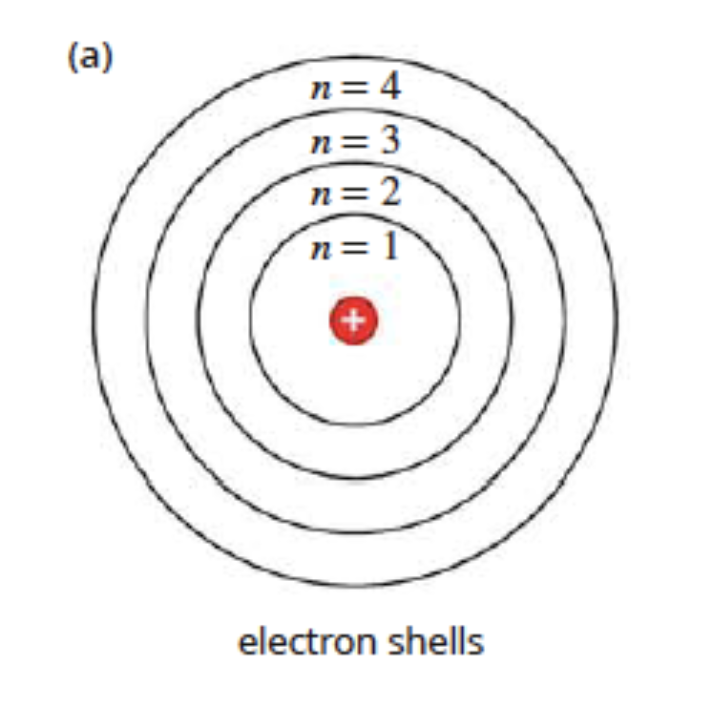
energy levels increase the higher it goes
higher the gravitational pull, lower the energy
the electrostatic pull of the nucleus slows down the electrons
two electrons in the same electron shell (energy level) have anticlockwise spins, aka they circle around each other at the same speed meaning that they will never meet
(insert diagram)
all elements’ electrons orbit in the energy levels at the same speed.
Different Atoms
the type of atom that makes up each element is determined by the number of protons (atomic number) in the nucleus (protons determine electron identity)
atomic number: the number of protons in the nucleus of the atom
mass number: the total number of protons + neutrons in the nucleus
atoms are electrically neutral: therefore number of electrons = number of protons
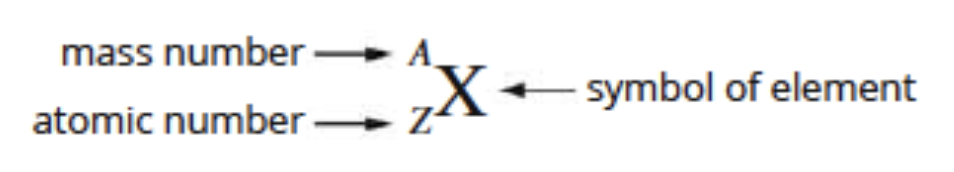
(the periodic table shows the most common isotopes)
Calculating the Number of Substance Mass
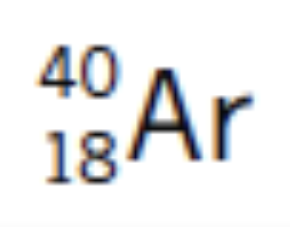
example atom: argon
the number of protons = Z = 18
the number of neutrons = A - Z = 22
the number of electrons = Z = 18
protons determine elemental identity
electrons determine chemical reactivity
(ability of an atom to undergo chemical change in a chemical reaction)
neutrons determine the physical properties of an element
Isotopes
all atoms that belong to the same element have the same number of protons in the nucleus and therefore the same atomic number, Z
atoms that have the same number of protons (atomic number) but different number of neutrons (and therefore different mass numbers) are known as isotopes
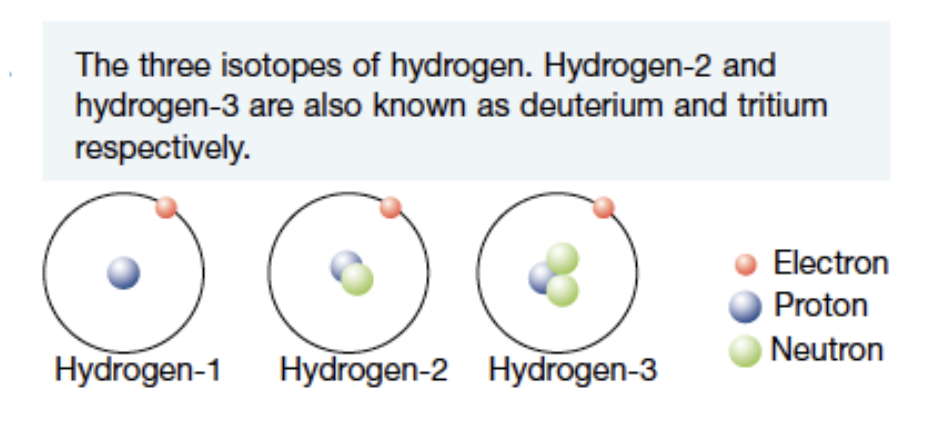
isotopes have identical chemical properties but different physical properties such as mass and density
some isotopes are radioactive
Electron Configuration
Electron Configuration - Bohr Model
using the bohr model, it is possible to determine the basic electron configuration of any atom by applying the following rules:
rule one: each shell can only contain a certain maximum number of electrons
electron shell
maximum number of electrons
1
2
2
8
3
18
4
32
n
2n²
rule two: lower energy shells fill before higher energy shells
these rules only really fully apply to the first 1-18 elements
for any element with an atomic number of >18 → shell 3 holds 8, no more 2n² until really heavy elements (start in transition metals)
Worked Example
step one determine the number of electrons in sodium (Na) | 11 electrons |
step two recall the maximum number of electrons each shell can hold | 1- 2 2- 8 3- 18 |
step three place the 11 electrons in the shells from the lowest energy to the highest energy. do not exceed the maximum number of electrons allowed | 1- 2 2- 8 3- 1 |
step four write the electronic configuration by listing the number of electrons in each shell, separated by commas | 2, 8, 1 |
Flame Test Theory
energy is the ability to do work and comes in a variety of forms, for example heat, light, motion, electricity, etc
when atoms are given energy (in this case, heat energy), the electrons jump up to a higher energy level
work-changing matter
chemical state (i.e. in a chemical reaction (process where atoms are rearranged so that bonds are broken and new))
movement
physical state (liquid, gas, solid
e.g. kinetic energy is the ability to move matter (work)
acceleration
deceleration
stop moving
change direction
e.g. potential energy is the ability to store energy (work)
e.g. gravitational energy is the ability to pull objects with smaller mass to objects with bigger mass (work)
e.g. thermal energy is the ability to combust matter, excite electrons (work)
e.g.
explanation: in n=1, electrons are moving around with 1J (joule), and in n=2, they are moving with 3J of energy, so the electrons need 2J from the flame to move from n=1 to n=2
oxygen is used to combust orgainic matter. as long as there is oxygen, any organic matter can combust
Ions
Ions
remember: protons have a charge of +1, electrons of -1
a positively or negatively charged atom or group of atoms
monoatomic: Na+, Cl-, Cu+2
polyatomic: Po3-, OH-
charged particles which form when an electrically neutral atom gains or loses electrons
this causes to an imbalance, leading to a positively or negatively charged ion
Cations (🐈⬛) and Anions (🧅)
when an atom loses electrons it becomes positively charged, or, a cation
e.g. Mg2+, Al3+
when an atoms gains electrons it becomes negatively charged, or, an anion
e.g. Cl-, O2-
a neutrally charged atom has an equal amount of protons (+) and electrons (-)

Octet Rule
the octet rule is the tendency on atoms to prefer to have 8** electrons in their valence shell*
this is because this is a stable arrangement, and equivalent to a full shell
when given the opportunity, atoms will gain, lose or share electrons to follow the octet rule
this forms bonds (ionic, covalent, metallic (will be covered later))
* the valence shell is the outermost electron shell
** for hydrogen and helium, the octet rule does not apply, and instead they must have 2 electrons in their valence shell to achieve stability
Cation or Anion?
atoms always take the path of least resistance
this means that if, for example, an atom has two in their valence shell, they will loose two atoms, and if they have six, they will gain two atoms
all metals will become cations, all non-metals will become anions
if the transition metals are removed, then the remaining groups are numbered 1-8, \ representing the number of electrons in their atoms’ valence shells (excluding He)
groups 1-3 all shed electrons, while groups 5-7 all gain electrons
group 8 elements are non-reactive, as they already have a full valence shell
group 4 metals do not give or take, but instead share, forming covalent bonds (more on this later)
Naming Monoatomic Ions
cations: refer to the metal name
e.g. Al3+ → Aluminium Cation
anions: replace the suffix with ‘-ide’
e.g. O2- → Oxide
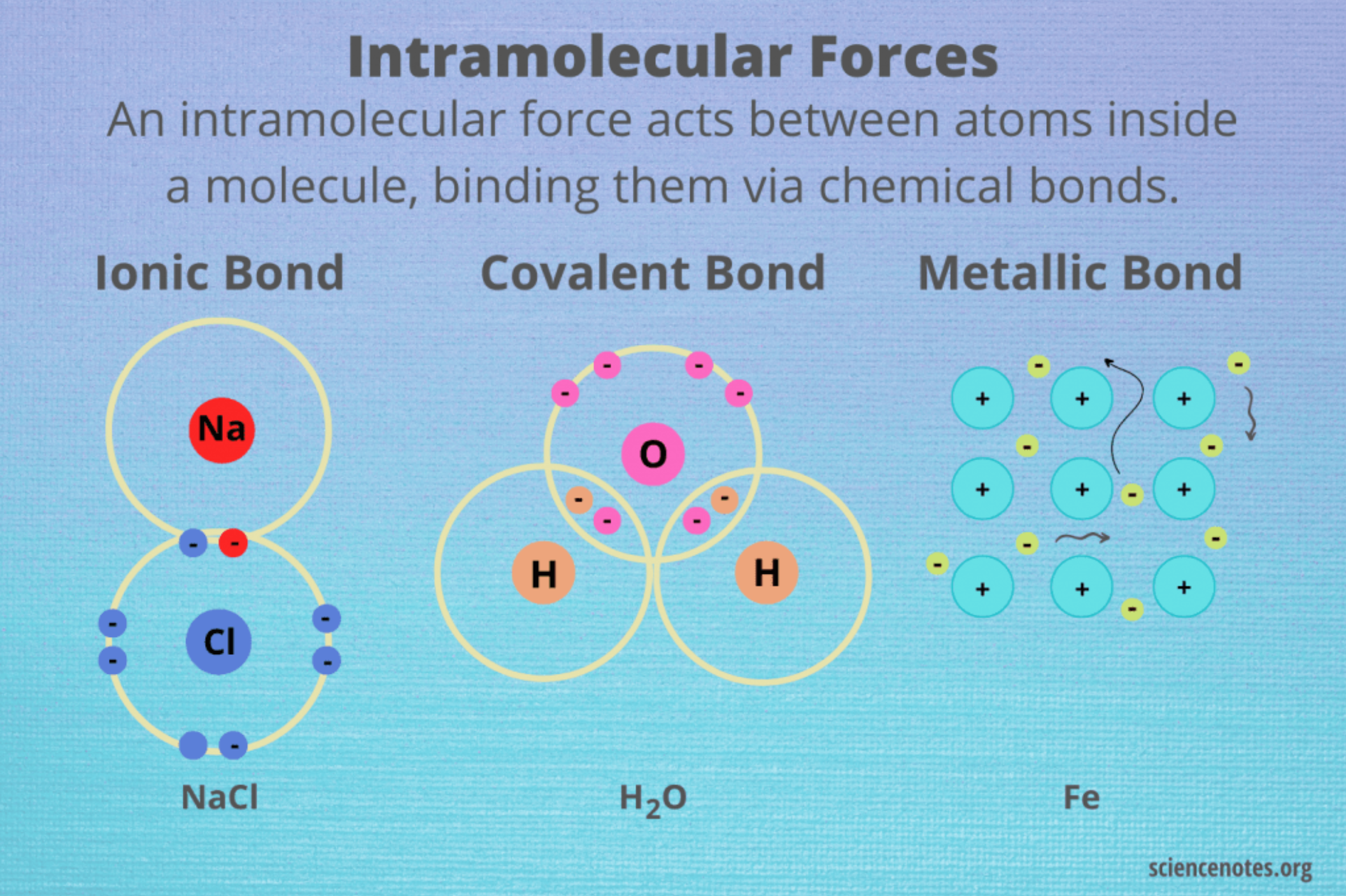
Electron Transfer and Ionic Bonding
Ionic Compounds
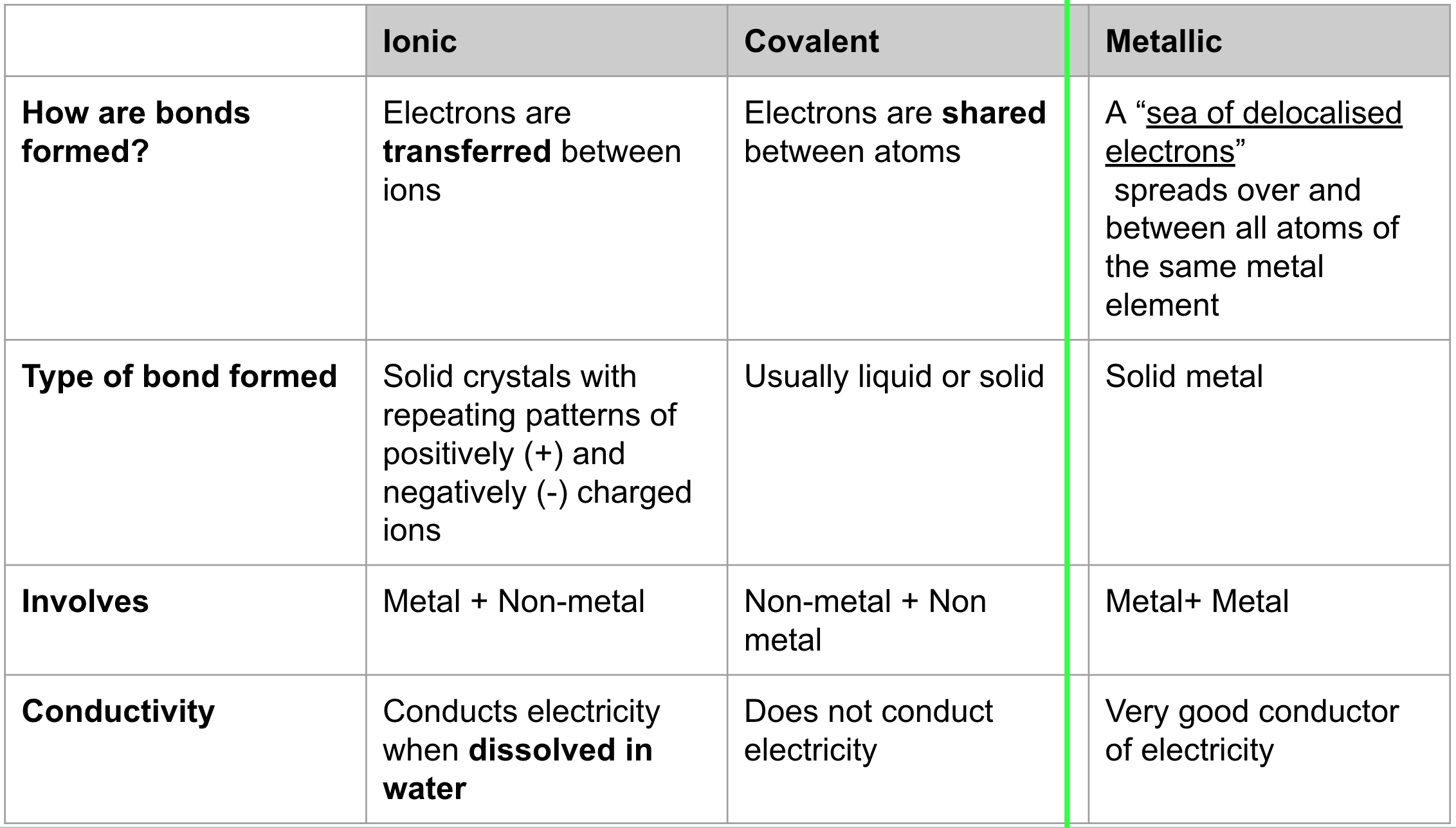
always formed with a metal and non-metal
metallic cation + non-metallic anion = ionic compound
during a reaction, there is a transfer of electrons (Metal → Non-metal)
once this occurs, the oppositely charged ionic bond together in a lattice
ionic bond = electrostatic attraction between a cation and anion
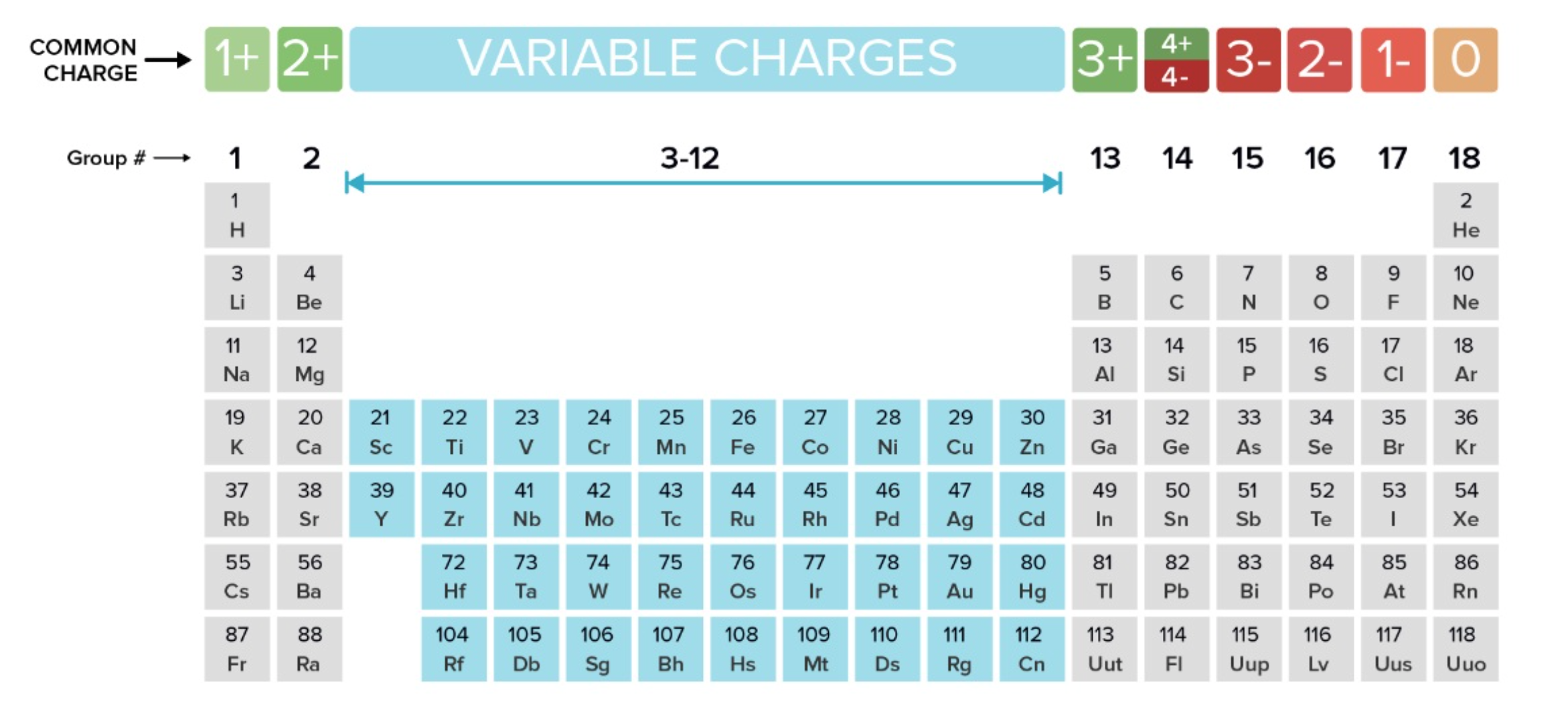
Lattice Structure
made up of cations and anions side by side
opposites touching only (✨opposites attract✨)
e.g. sodium chloride (aka table salt)
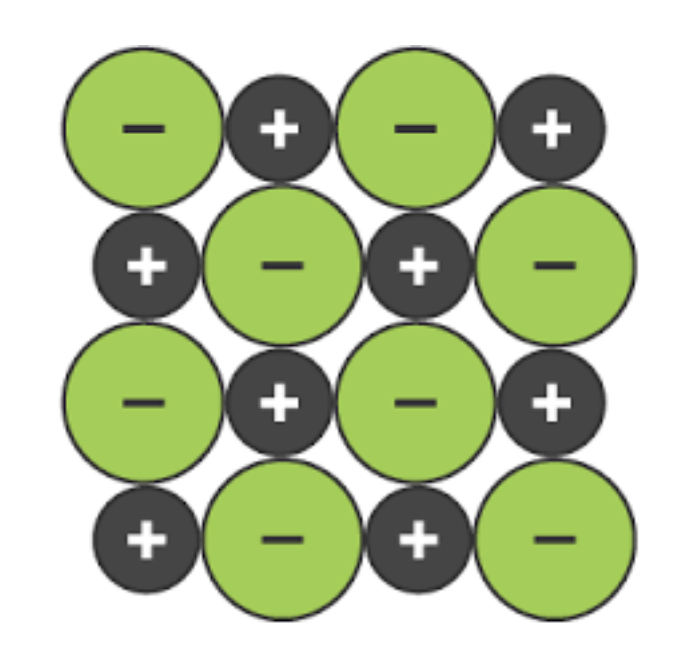
no. cations = no. anions
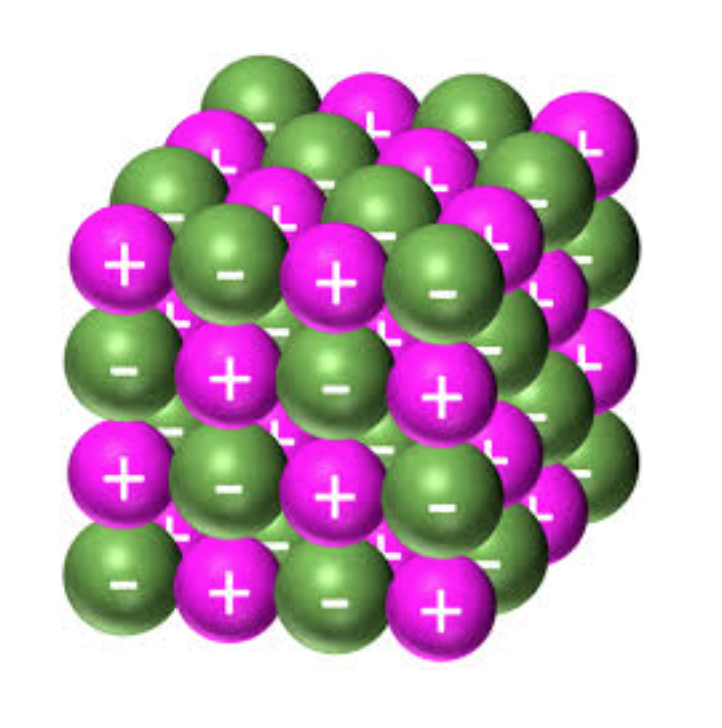
ionic compounds are brittle, as when a force is applied to the lattice, the ions with like charges align and actively repel each other, shattering the lattice
Different Models of Ionic Bonding
Model | Example | Does Not Show |
Chemical Formula | NaCl |
|
Dot and Cross Diagram | 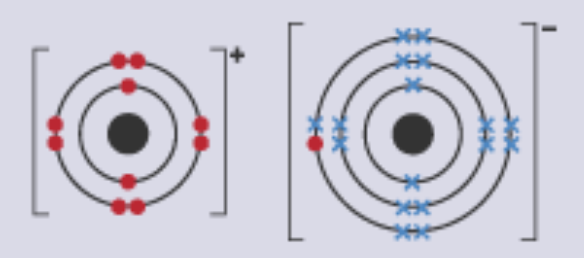 |
|
2D Diagram | 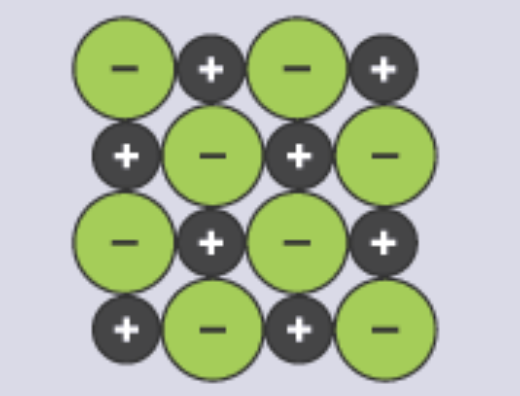 |
|
3D Diagram | 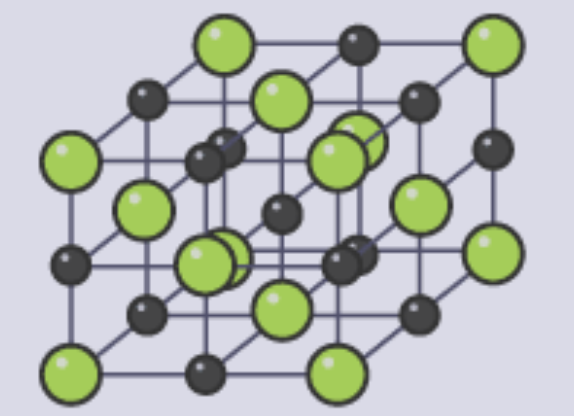 |
|
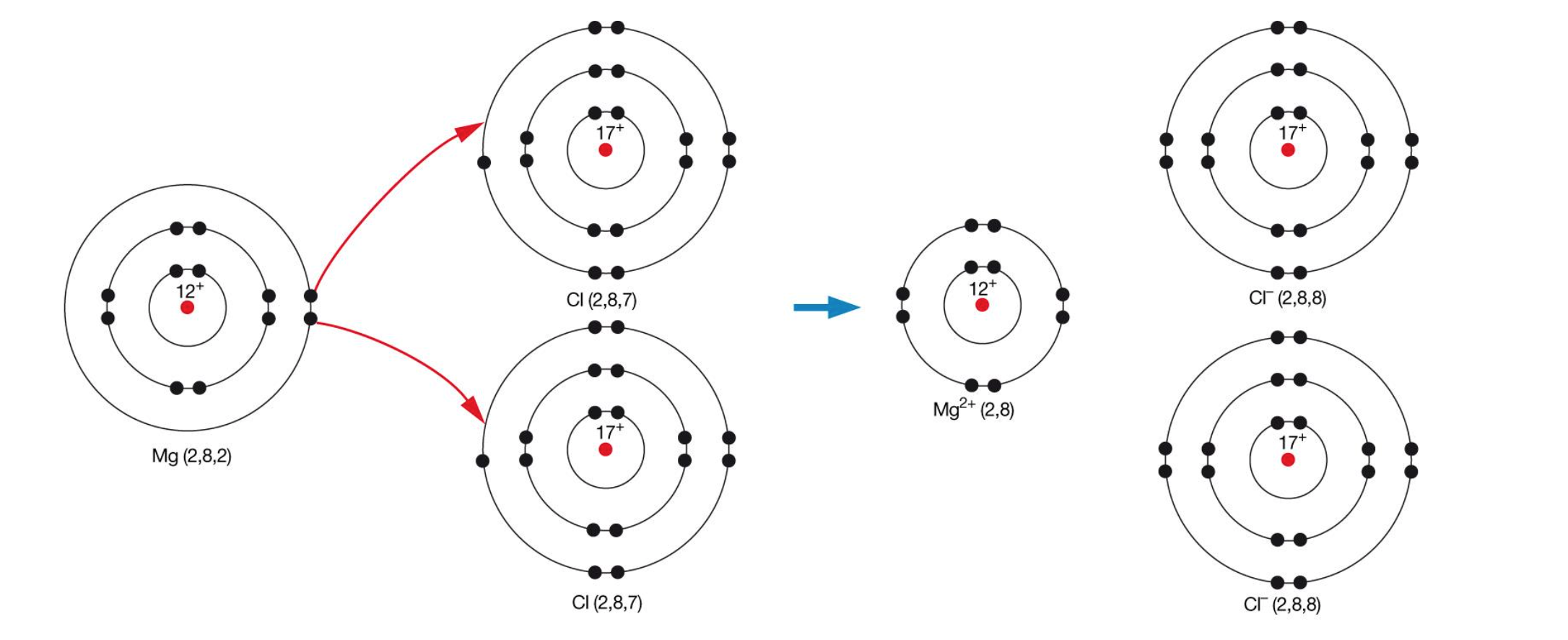
Electron Transfer Diagrams
used the show the path that electrons take when they are removed from a metal and added to a non-metal during ionic bonding
e.g.
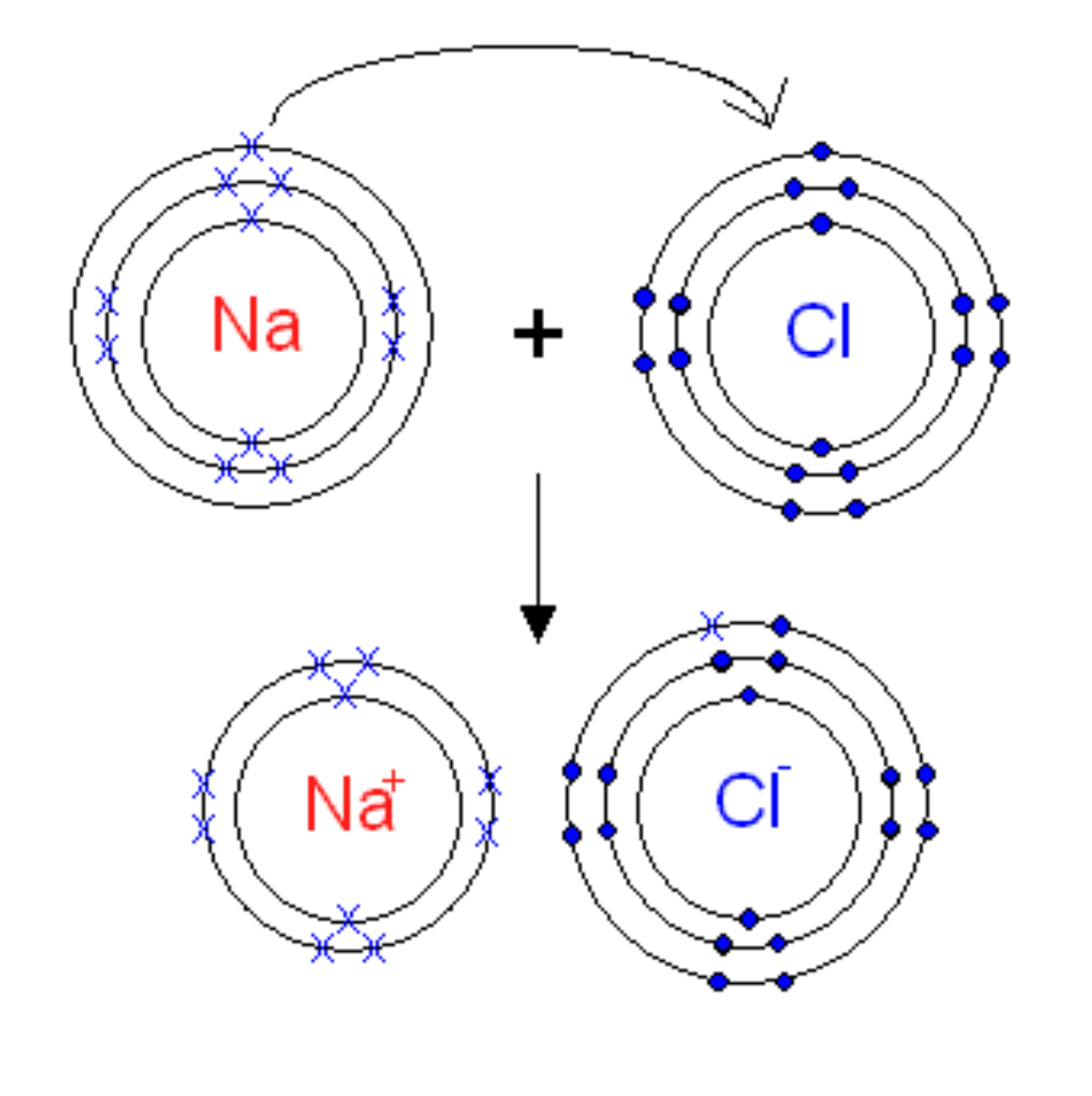
steps
1- draw an electron shell diagram of the neutral metal and non-metal
2- add a ‘+’ between them
3- draw an arrow leading from each valence electron in the metal to the valence shell
of the non-metal
4- add an arrow toward the resulting ions
5- draw an electron shell diagram of the resulting cation/s and anion/s
6- write the chemical symbol of the metal and the non-metal in the centre of the
electron shell diagram, taking care to add charges to your ions
Naming Ionic Compounds
Rules
name cations (metals) first before anions (non-metals)
extension- if the metal is a transition metal, indicate the valency in numerals after the name (e.g. Fe(III), Ag(I), Gold(I))
the name of the metal cation remains as is (e.g. Sodium ion, Na+ ion)
the name of the non-metal is changed. it’s suffix becomes ‘-ide’
Na+ (cation) + Cl- (anion) = NaCl (sodium choride)
Rules for Writing Chemical Formulas (e.g. Lithium Oxide)
write the symbol and charge of the two ions forming the ionic compound - Li+, O²-
calculate the lowest common multiple of the two numbers in the charges of the ions - 1×2=2
calculate how many cations and anions are needed to equal the lowest common multiple - two Li+ ions, one O²- ion
determine the formula of the ionic compound write the symbols of the cation first - Li2O
the number one should not be written in subscript
shortcut:
Li1+|\ O2-|/
Li2O
Naming Ionic Compound
the name of ionic compounds are written by listing the name of the positive ion followed by the name of the negative ion. a space separates the two parts of the ionic compound name
e.g. NaCl → sodium chloride
e.g. K2O → potassium oxide
e.g. CaH2 → calcium hydride
if the cation element has more than one possible oxidation state (charge), follow the element name by parenthesis containing the approximate Roman Numerals
e.g. Fe2O3 → Fe3+, O2- → Iron (III) oxide
there is no prefix indicating the number of atoms in the cation. so Hg2Cl2 is mercury(II) dichloride
* Ca2+, O2- → CaO (simplest form) CaO (wrong)
* x4+, y2- → xy2 (simplest form) x2y4 (wrong)
examples:
K2O → Potassium Oxide (K+, O2-)
NaOH → Sodium Hydroxide (Na+, OH-)
CaBr2 → Calcium Bromide (Ca2+, B-)
Al2S3 → Aluminium Sulfide (Al3+, S2-)
Li3N → Lithium Nitride (Li+, N3-)
Be(NO3)2 → Beryllium (Be2+, NO31-)
Metallic Bonding
Metallic Bonding
only made up of metals (cations)
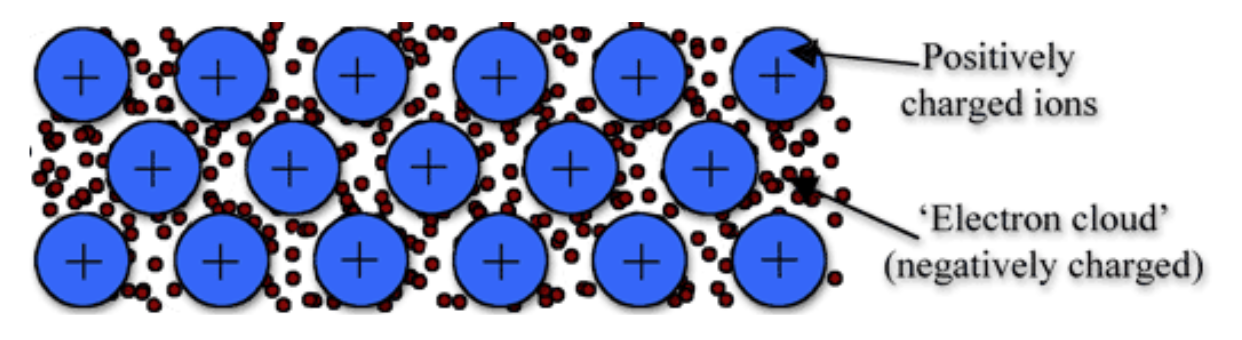
cations shed valence shell electrons
delocalised sea of electrons (free moving electrons)
Structure: Crystal Nature of Metallic Bonding
metals occur as crystal lattices
this is because metallic atoms tend to lose their outer shell electrons easily
this turns the metal elements into positively charged cations
these cations form a metallic lattice structure, in which electrons from each metal atom overlap with each other, forming a sea of electrons that can flow between all the metal ions.
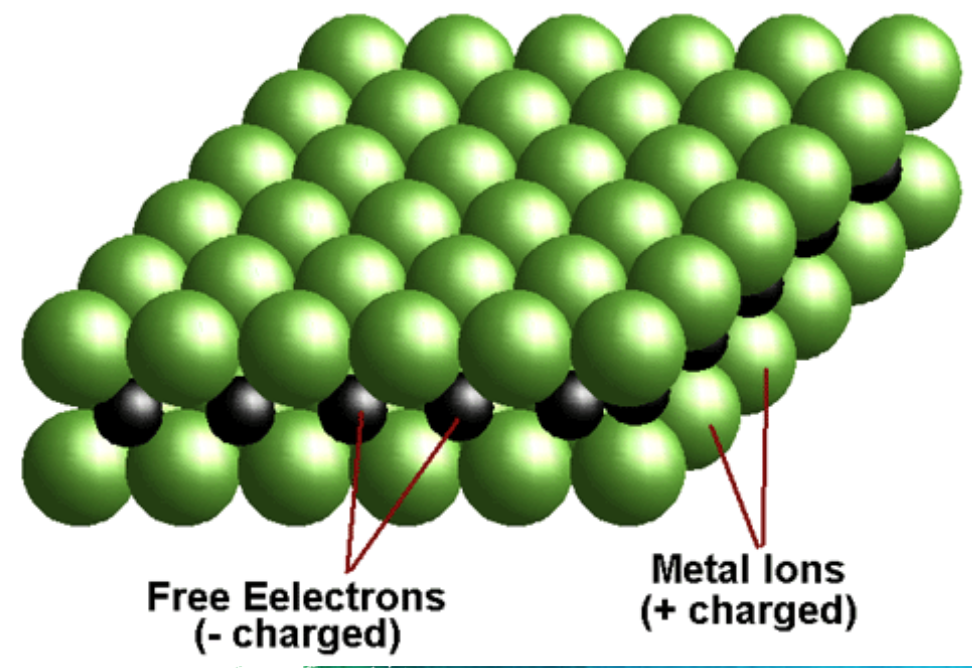
electrostatic forces of attraction between the positively charged metal cations and negatively charges valence electrons occur in all directions, holding the lattice together
this type of non-directional bonding is known as metallic bonding
this means that metal atoms are hard to seperate but relatively easy to move (malleable)

Alloys
alloys are a mixture of two or more elements where one element is a metal, combined via metallic bonding
alloys are generally harder than the pure elements they contain. this is due to the pure metal atoms being the same and arranged in layers, as apposed to alloys which contain elements with atoms of different sizes
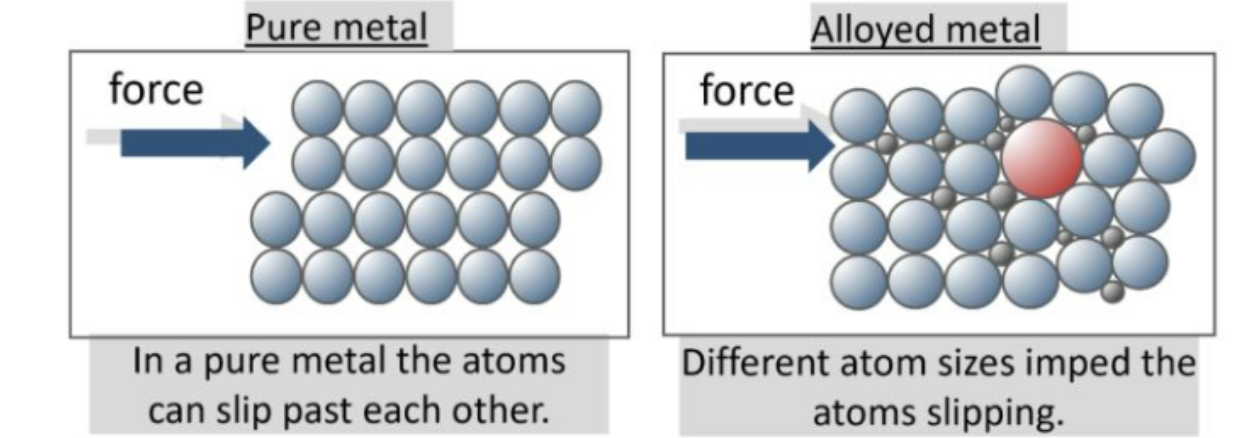
e.g. steel- iron (metal) + carbon (non-metal)
e.g. bronze- copper (metal) + tin (metal)
e.g. brass- copper (metal) + zinc (metal)
Types of Bonding
metal + metal = ionic compound
non-metal + non-metal = covalent compound
metal + metal = metallic mixture
How to Form a Lattice
metals → “big granular chunks” (e.g. sodium → big, soft, reactive)
+ non-metals → often a gas (e.g. chlorine → liquid)
millions of atoms combine at high temp
Metallic vs Ionic Compounds
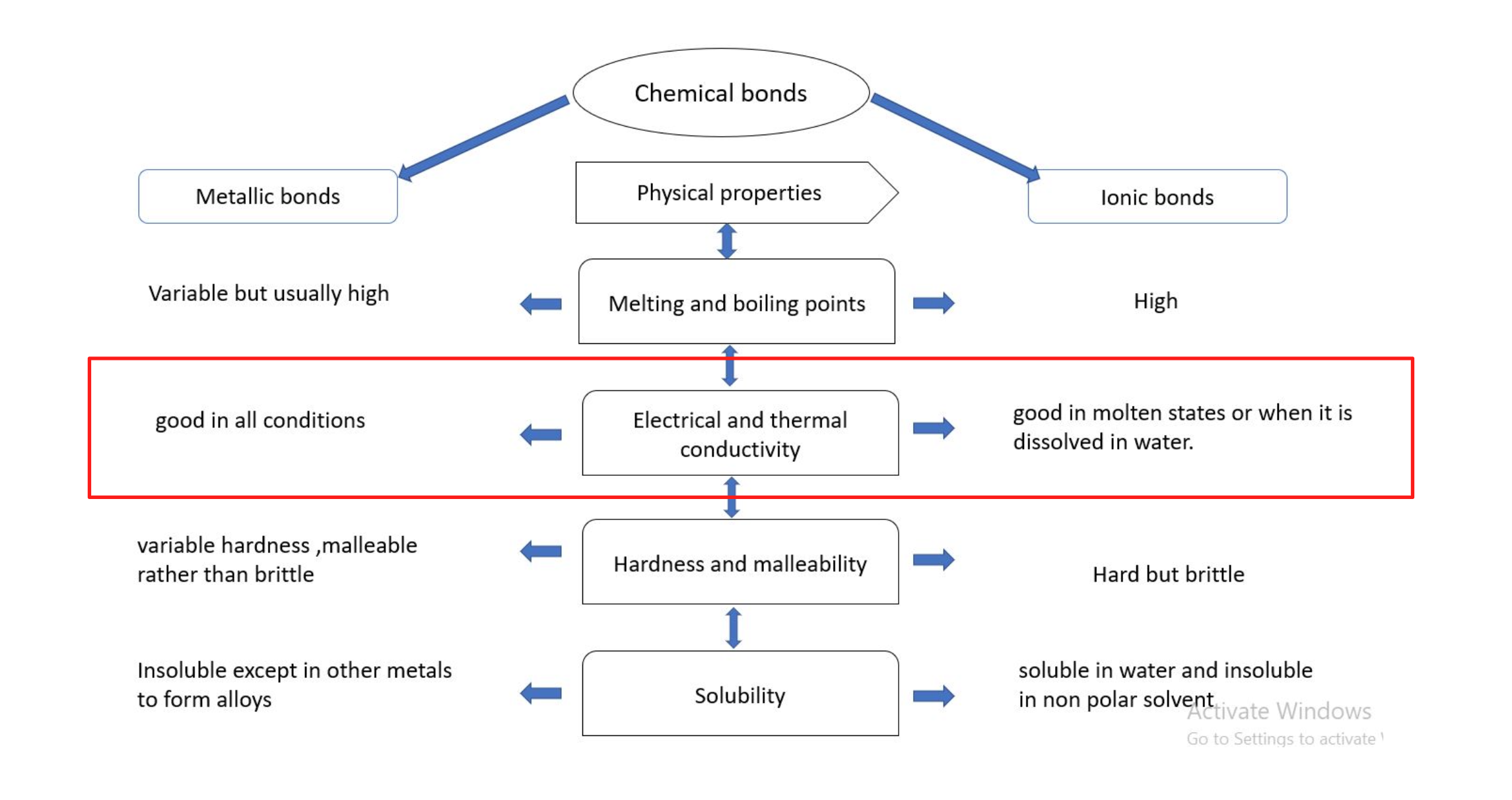
metals are good conductors of electricity