rate determining step and arrhenius eqn
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
what is the rate determining step?
slowest step of a reaction mechanism
determines overall rate
how can we use evidence from the rate equation to explain why a step is the RDS:
species [X] (or more) is in the rate equation and in step 1
so step 1 must be the RDS
![<p>rate = k[B]<sup>2</sup>[H<sup>+</sup>]</p>](https://knowt-user-attachments.s3.amazonaws.com/1a2fe4d6-496e-4faf-b11f-e5f94e79e4f8.png)
rate = k[B]2[H+]
step 2
by the end of step 2, 1x H+ and 2x B have been used (as seen in the rate eqn)
what does the Arrhenius eqn show?
shows the effects of changing the temp/Ea of the reaction on the rate constant
give the standard form of the Arrhenius eqn and explain what each of the values represent:

give the logarithmic form of the Arrhenius eqn and explain what each of the values represent:
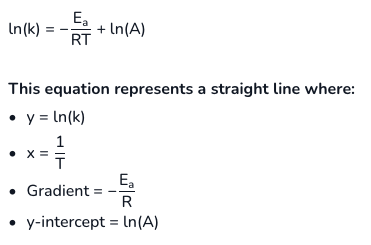
how does Ea affect the rate constant?
as Ea increases, k decreases
as the higher the Ea, the fewer reactant particles will have sufficient E to react
so fewer successful collisions occur
this means a reaction w/ high Ea has a lower RoR
how does temperature affect the rate constant (k)?
temp increase → exponential increase in k
EK of particles increases and particles move faster
so a higher proportion of particles have an energy at/above the EA
so there is a higher frequency of successful collisions
so RoR increases
explain why increasing temp has a much greater effect on RoR than increasing [E]:
reaction occurs when molecules have E ≥ Ea
increase temp causes many more molecules to have this E (sig higher fraction of molecules have Es ≥ Ea)
so higher prop of collisions become successful and RoR increases more
whereas increasing [E] only increases no. w/ this E