BSCI170: Metabolism and Enzymes
1/33
Earn XP
Description and Tags
notes from 10/20 + 10/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
What is metabolism, and what are it’s functions?
total chemical reactions of an organism
Maintains stability of the cell
Provides building blocks for the synthesis of new cellular materials
Highly organized and controlled
What are nutrients, and give some examples
supply of monomers needed for cell growth
Examples of nutrients: CHNOPS
What are metabolic pathways, and what are their characteristics?
Metabolic pathways: series of interconnected reactions that convert a starting substrate into a product
Can be linear or cyclic
What is anabolism, and give an example
Anabolism: biosynthesis; uses energy to build molecules
Dehydration synthesis is anabolic
energy is consumed
What is catabolism, and give an example
Catabolism: digestive; releases energy to break down molecules
Hydrolysis is catabolic
energy is released
ATP hydrolysis turns ATP into…
ATP → ADP + pi
Catabolic
You make energy by breaking ATP bc there is a lot of potential energy stored in the bonds
Why is ATP so important?
phosphate groups are very electronegative. As you add more phosphate groups, the bonds become increasingly unstable. Adding the third phosphate group allows it to break off easily, thus releasing energy
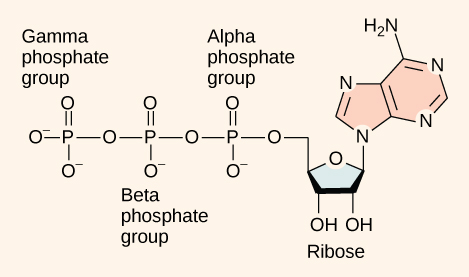
Naming of Phosphate groups in ATP
Alpha: phosphate group closest to the adenosine
Beta: 2nd closest
Gamma: 3rd closest
What is kinetic energy
energy of movement
potential energy
stored energy of objects
chemical energy
potential energy stored within chemical bonds
Gibbs Free Energy equation
ΔG = ΔH - TΔS
What is thermodynamics
study of energy and energy transfer involving physical matter
1st law of thermodynamics
energy of the universe is constant (energy can transferred and transformed but it cannot be created or destroyed)
2nd law of thermodynamics
the state of entropy of the entire universe, as an isolated system, will always increase over time (in energy transfer, some amount of energy is lost as heat)
High entropy equals…
high disorder = low energy
Low entropy equals…
low disorder = high energy
Gibbs free energy equation: ΔH
total energy in the system (enthalpy)
Gibbs Free energy Equation: ΔG
ΔG = change in free energy
Gibbs Free Energy Equation: ΔS
ΔS = amount of energy lost to entropy from the system’s total energy change
Gibbs Free Energy Equation: T
T = absolute temperature
ΔG of ATP
= -7.3kcal/mol
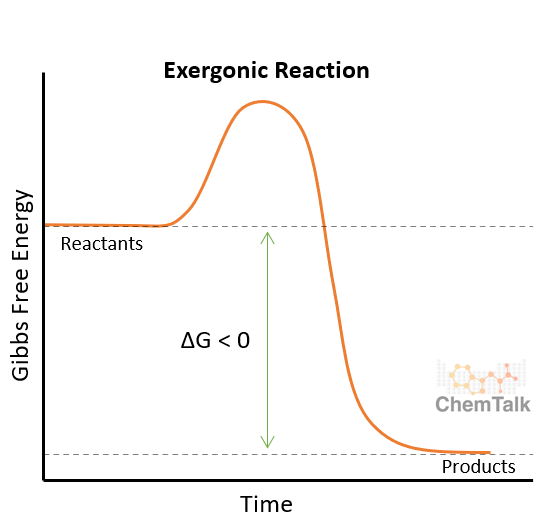
Exergonic reactions havea + or - ΔG
Reactions that release energy have a negative ΔG
Spontaneous: No energy inputted
Catabolic: breaks down molecules (this is how cells provide the energy needed for endergonic reactions)
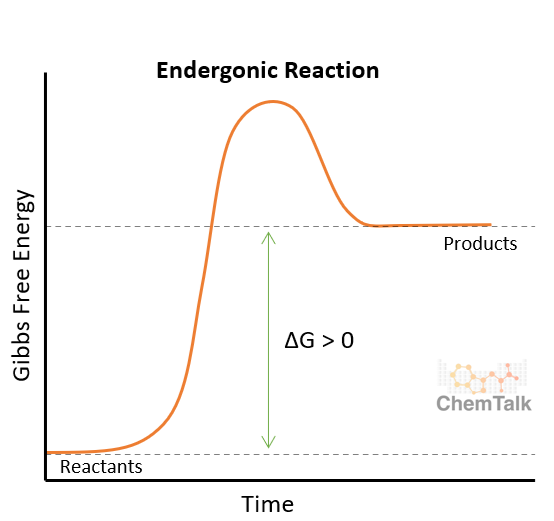
Endergonic reactions have a + or - ΔG
Reactions that absorb energy have a positive ΔG
Energy is inputted
Anabolic: makes molecules
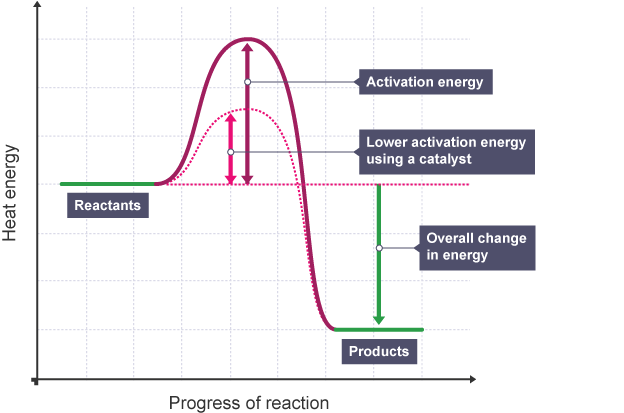
What is activation energy Ea
the amount of energy needed for a reaction to start
Impacts reaction rate
What is reaction rate?
measures how fast the reaction happens
Dependent on: temperature, concentration, and activation energy
what are catalysts
enzymes that lower activation energy
What are substrates
reactants/starting molecules
Enzymes bind to the active site of the substrate
Substrate changes shape once enzymes bind to it
Enzymes are…
Highly specific: they have a specific shape that allows them to only bind with a specific substrate
Reusable: can use them for reactions over and over again
Used in small amounts
Can be more than just proteins
What is the induced fit model?
each enzyme has a specific shape (lock) that allows a substrate (key) to fit and bind at the active site
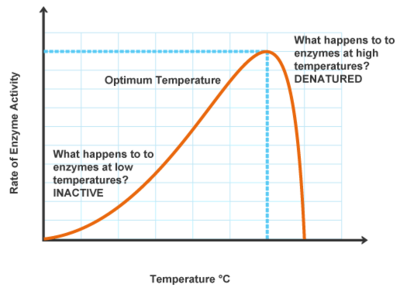
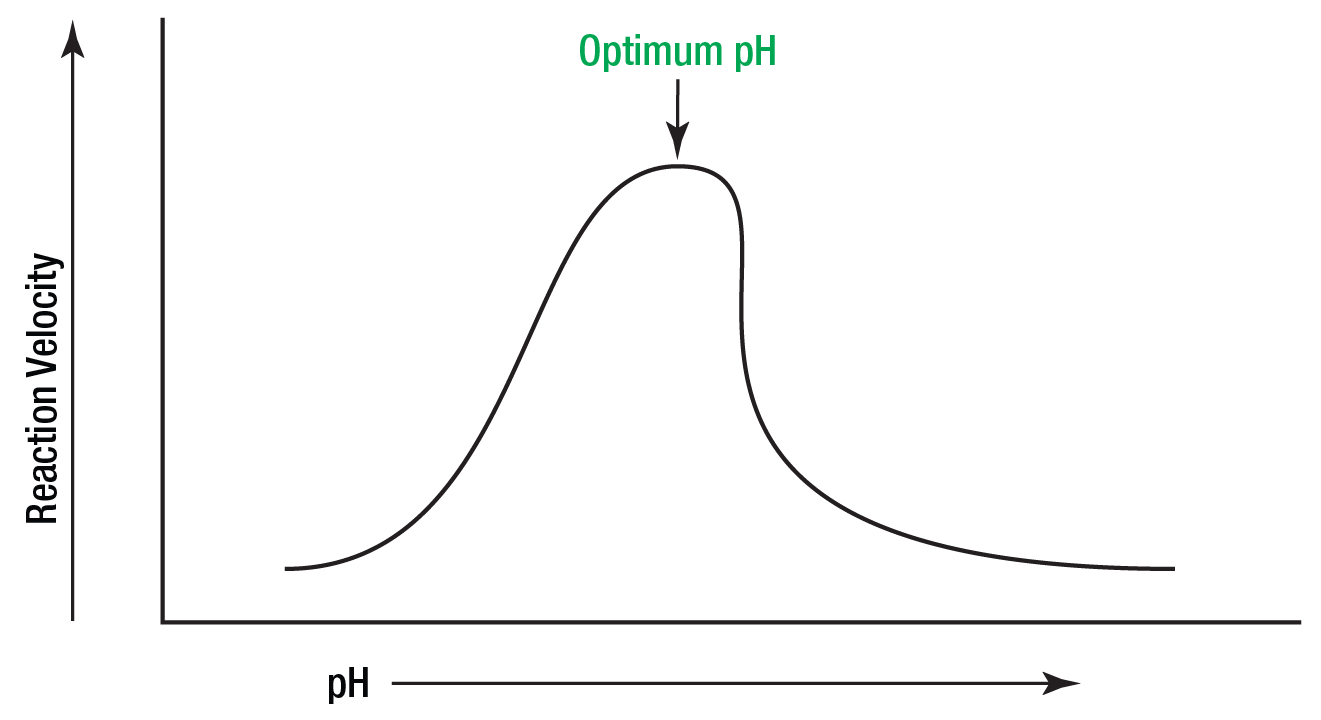
Denaturation of enzymes:
temperature
pH
concentration
Denaturation of enzymes: Temperature graph
optimal temp
Denaturation on Enzymes: pH
optimal pH
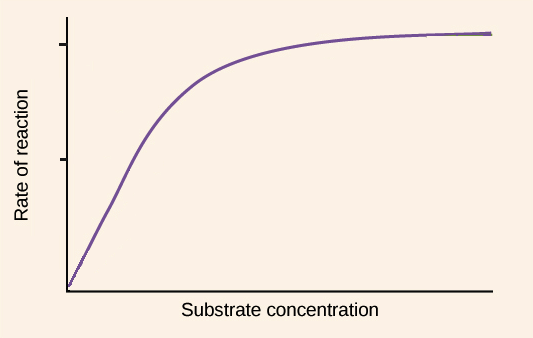
Denaturation of Enzymes: Substrate concentration
max concentration