Science revision!!Urgent!!!!!!
1/29
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Early atmosphere
The early atmosphere mainly consisted of mainly carbon dioxide,little to no oxygen and other gasses like ammonia.Theories say this is due to intensive volcanic activity at the time.
Modern atmosphere
The atmosphere has definitely changes,most of it being nitrogen,oxygen and carbon dioxide.Even though there is less carbon dioxide,climate change and the burning of fossil fuels adds onto the carbon footprint,increasing it again.
How oxygen increased
Oxygen levels in the atmosphere increased primarily due to photosynthesis by early plants and cyanobacteria, which produced oxygen as a byproduct, leading to the Great Oxidation Event.
carbon dioxide + water → glucose + oxygen.This process produces oxygen as a byproduct, increasing oxygen levels in the atmosphere.
How carbon decreased
Carbon dioxide levels decreased mainly through its absorption by plants during photosynthesis and oceanic absorption, which helped regulate the atmosphere's composition over geological time.
The greenhouse effect
The greenhouse effect is the warming that occurs when the Earth's atmosphere traps heat radiating from the Earth's surface, caused by greenhouse gases like carbon dioxide and methane.
Combustion of hydrocarbon fuels
The process of burning fossil fuels that releases carbon dioxide and other greenhouse gases into the atmosphere, contributing to climate change.
Complete combustion
is a reaction where hydrocarbons react with oxygen to produce carbon dioxide and water, maximizing energy release and minimizing pollutants.
Incomplete combustion
occurs when there is insufficient oxygen for the complete oxidation of the fuel, resulting in the production of carbon monoxide and other pollutants.
atmospheric pollutants
Substances released into the atmosphere that can harm human health and the environment, including gases like carbon monoxide and particulate matter. These pollutants can originate from various sources such as vehicle emissions, industrial discharges, and natural events like wildfires.
potable water
water that is safe for human consumption and use.
Desalination
is the process of removing salt and other impurities from seawater to produce fresh water suitable for drinking and irrigation.
distillation
a separation process used to obtain pure water by heating liquid to create vapor and then cooling the vapor back into liquid form. It effectively removes impurities and contaminants.
reverse osmosis
a water purification process that uses a semipermeable membrane to remove ions, molecules, and larger particles from drinking water, making it safe for consumption.
waste water treatment
is the process of removing contaminants and pollutants from wastewater to make it safe for discharge into the environment or for reuse. This includes physical, chemical, and biological methods to treat water.
Life cycle assessment
is a systematic analysis of the environmental impacts of products or services throughout their entire life cycle, from raw material extraction to production, use, and disposal.
Recycling
is the process of collecting, processing, and reusing materials that would otherwise be thrown away as trash, helping to conserve resources and reduce environmental impact.
The history of atoms,john dalton
the study of the development of atomic theory, starting with John Dalton's proposal in the early 19th century that matter is made up of indivisible particles called atoms, paving the way for modern chemistry.
The history of atoms,mendeleev
the study of Dmitri Mendeleev's contributions to atomic theory, particularly his formulation of the Periodic Law and the development of the periodic table, which organized the known elements based on their atomic weights and properties.
The history of atoms,rutherfords gold experiment
Ernest Rutherford's gold foil experiment, which demonstrated that atoms have a small, dense nucleus surrounded by mostly empty space, leading to the understanding of atomic structure.
Models of atoms
the conceptual frameworks developed to understand atomic structure and behavior, including Dalton's model, Thomson's Plum Pudding model, Rutherford's nuclear model, and Bohr's planetary model.
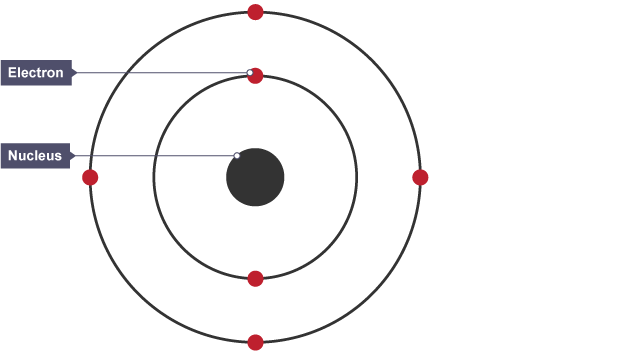
The structure of an atom
refers to the arrangement of protons, neutrons, and electrons within an atom, defining its chemical properties and behavior.
Subatomic particles
the fundamental constituents of an atom, including protons, neutrons, and electrons that determine its properties and behavior.
subatomic particle relative mass and change
(order of mass,then charge)
Electrons: very small,-1
Protons:1,+1
Neutrons:1,0
atomic mass and number
refers to the mass of an atom as determined by the number of protons and neutrons in its nucleus, and its atomic number indicates the number of protons contained in the nucleus.
Isotopes
variants of a chemical element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses.
calculating relative atomic mass
determining the weighted average of the isotopes of an element based on their relative abundances.
The modern periodic table
elements are arranged in rows,called periods in order of increasing atomic number.Elements with similar properties are placed in vertical columns,called groups
Group 1,Alkali metals
The alkali metal are a group of metals whcih are highly reactive with water. As you go down,they get more and more reactive with water and form hydroxides. They all have to be stored in oil,so they do not react with anything around them.These elements for metal hydroxides which are alkaline.
Group 7,halogens
Halogens, which are nonmetals known for their reactivity and tendency to form salts with metals. They exist as diatomic molecules and become less reactive as you move down the group. They have seven electrons in their outer shell,making them less reactive as you go down the group.They form salts when they react with metals
Group 0(8),noble gases
Noble gases are colorless, odorless gases that are very unreactive due to having a full outer shell of electrons. They include helium, neon, argon, krypton, xenon, and radon.