carbonyls part 2
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
why are imines less reactive towards nucleophiles than carbonyls?
N is less electronegative than O
LUMO is higher in energy so less accessible
what does it mean if N is less electronegative than O? (negative charge)
harder to put a negative charge on N than O
why are iminium ions more reactive towards nucleophiles?
ions are positively charged and have much lower LUMOs than neutral carbonyl groups
what is the order of reactivity towards nucleophiles for imines, carbonyls, iminium ions?
imine < carbonyl < iminium
what do imines form after nucleophilic addition?
high energy amide anion
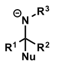
what do carbonyls form after nucleophilic addition?
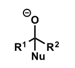
what do iminium ions form after nucleophilic addition?
stable neutral amine
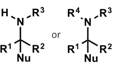
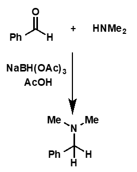
what is the product of a primary/secondary amine and carbonyl group? what is also needed?
what is the name of this reaction?
suitable reducing agent
leads to substituted amines
reductive amination
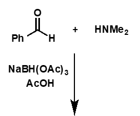
what is the product of this reaction?
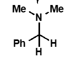
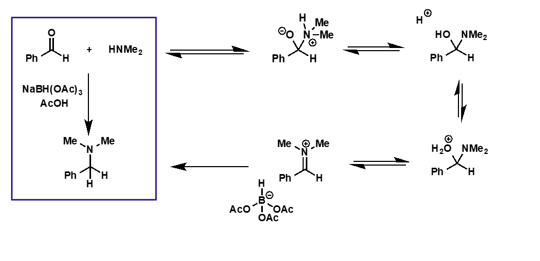
what is the mechanism for this reaction?
hint - extra H comes from reducing agent
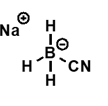
what is the structure of sodium cyanoborohydride
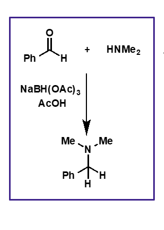
for reductive amination, what type of reducing agent is needed? why?
mild reducing agent which cannot reduce the carbonyl before the iminium ion is formed
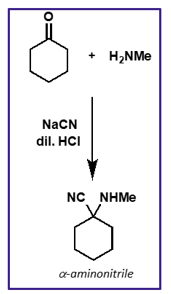
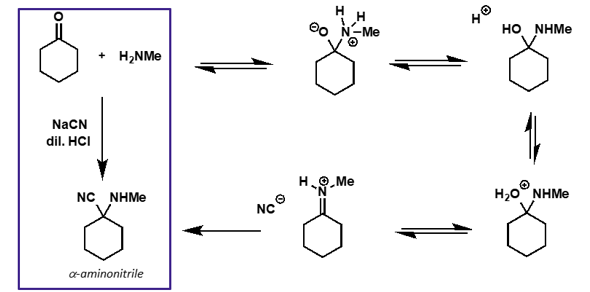
what is the mechanism for this reaction?
what is cyanide acting as?
cyanide is a nucleophile
what is the reagent?
LiAlH4
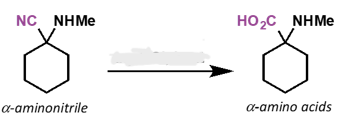
what is the reagent?
aq. HCl , heat
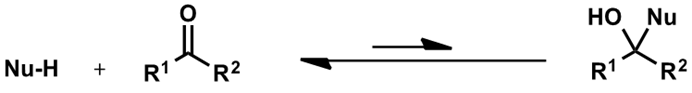
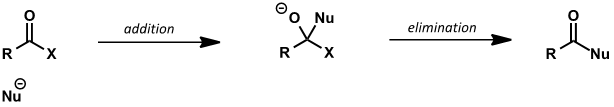
what is the equilibrium between Nu, carbonyl and tetrahedral intermediate?
which way does it lie?
what is a carboxyl derivative?
C/H on ketone/aldehyde is replaced by heteroatom
how does carboxyl equilibrium with Nu differ from carbonyl?
there is a leaving group on the tetrahedral intermediate = possible elimination reaction
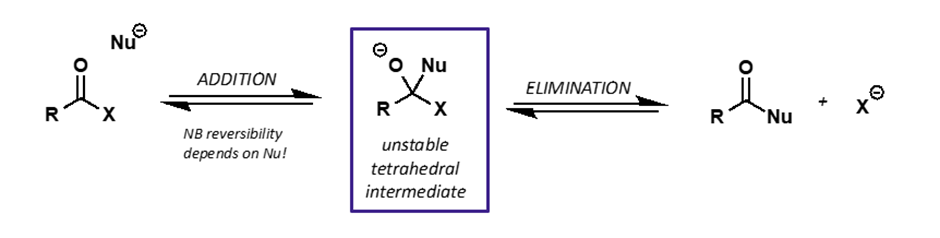
what to remember when drawing addition/elimination mechanism for carboxyl groups?
it is not one step, otherwise would be SN2
what is reaction for esterification of carboxylic acid + alcohol?
catalysed by acid
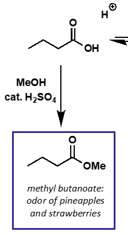
what is mechanism?
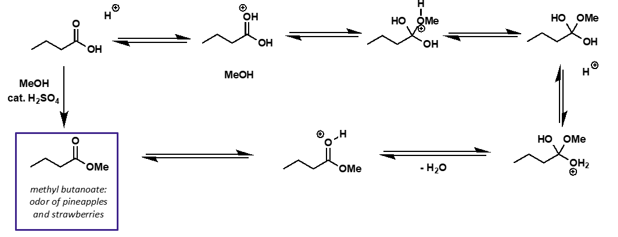
why is esterification of carboxylic acids roughly thermoneutral?
what does this mean for the equilibrium?
esters and acids are similar in structure and bonding
the equilibrium is central - would be a mixture of reagents and products
how can the equilibrium of c. acid + alcohol = ester be shifted?
using alcohol in large excess (volatile alcohols eg methanol or ethanol as solvents)
driving off water
how does industrial process of condensation polymerisation shift equilibrium?
high temperatures drives of water as steam
is a catalyst needed for esterification from an acid halide?
no as acid chlorides are much more electrophilic
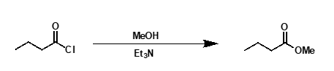
what is the mechanism?
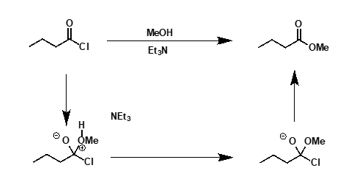
why is acid chloride + alcohol = ester automatically shifted to the right?
chloride is a good LG and not particularly good nucleophile so formation of ester is favourable
what other chemical is needed for acid chloride esterification?
non nucleophilic base to neutralise acidic by-product
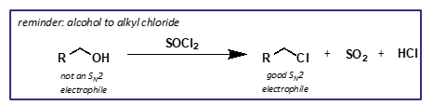
how are alkyl chlorides synthesised?
from alcohol and thionyl chlorides
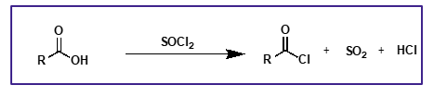
how are acid chlorides synthesised?
carboxylic acids and thionyl chlorides
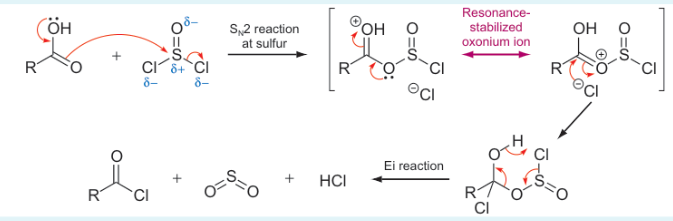
what is mechanism?
why are acid halides more reactive than acid anhydrides in forming esters?
chloride much better leaving group than acetate ( - 3 vs 5 pKa)
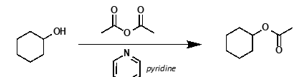
what other chemical is used for esterification by acid anhydride?
what is it acting as?
pyridine
acting as base and nucleophilic catalyst
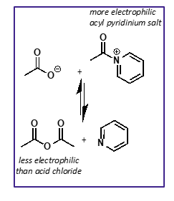
how is pyridine involved in acid anhydride reaction?
forms acyl pyridinium salt
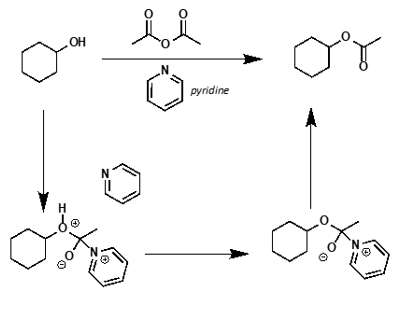
what is mechanism?
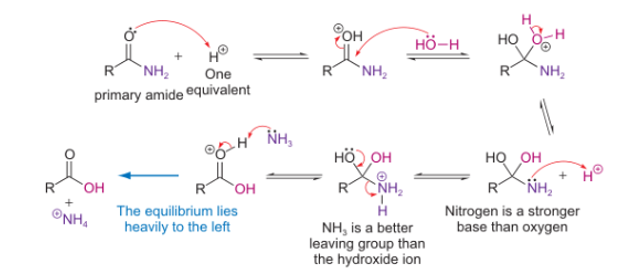
can amides be formed from carboxylic acids and amines?
no
incompatibility of reactants at any pH
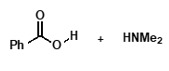
under neutral conditions?
unreactive protonated amine and unreactive carboxylate ion
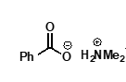
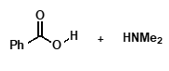
under acidic conditions?
protonated amine is formed, nucleophilicity is lost
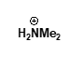
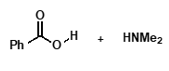
under basic conditions?
unreactive carboxylate ion due to negative charge
what is needed when amide is made from acid chloride?
hydrogen chloride made as by product
base needed to neutralise - can be excess amine nucleophile
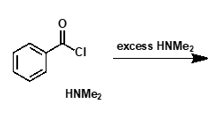
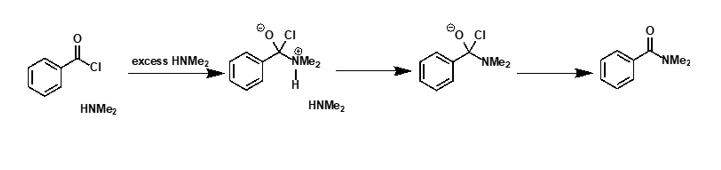
what is mechanism?
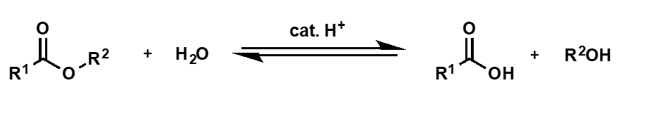
ester hydrolysis (catalysed by acid) mechanism
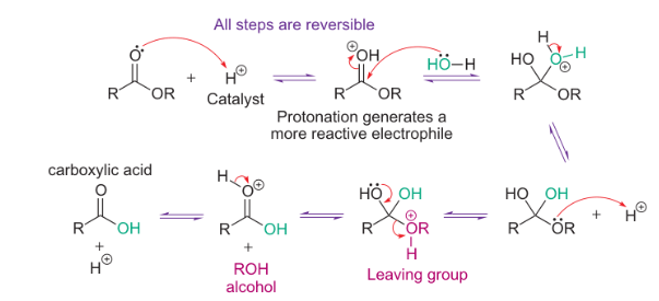
why is ester hydrolysis more favourable under basic conditions?
equilibrium displaced towards products
favourable deprotonation of initially formed carboxylic acid
how to isolate c. acid after basic ester hydrolysis?
protonation by strong mineral acid
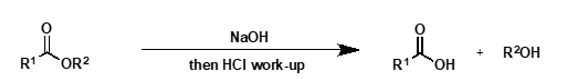
what is mechanism for basic ester hydrolysis?
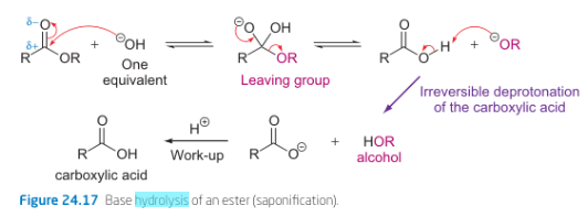
what are conditions for amide hydrolysis?
can be acidic or basic
reactions require more forcing than for esters - higher temperatures, more concentrated acid/base, longer reactions
amide hydrolysis acidic mechanism
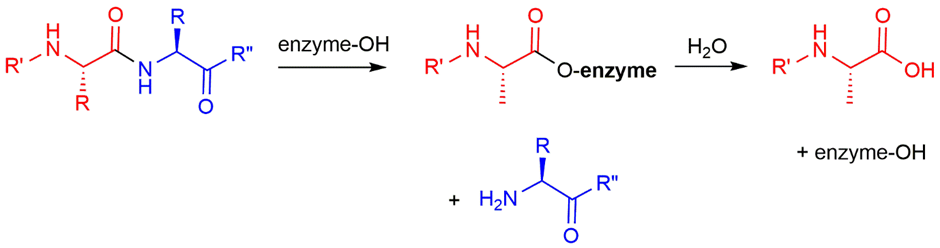
how do enzymes catalyse the peptide hydrolysis?
nucleophilic alcohol that reacts with amide to form ester
enzyme groups stabilise tetrahedral intermediates in both steps
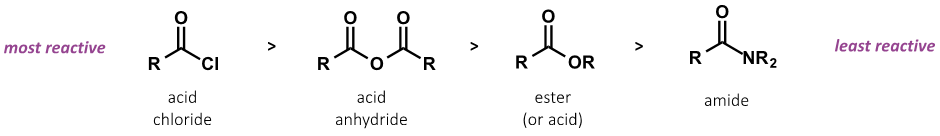
what is trend in reactivity of:
amide, acid anhydride, ester/acid, acid chloride
for rates of nucleophilic substitution
what is the rate determining step?
either can be
what does rate of second step depend on?
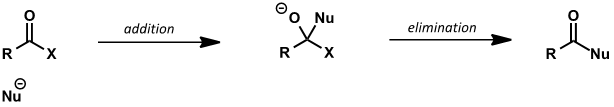
leaving group ability
what is trend in leaving group ability for carboxyl groups? (acid chloride, acid anhydride, ester/acid, amide)
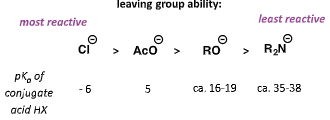
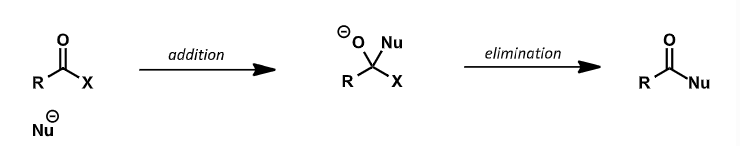
what does rate of first step depend on?
how electrophilic the carbonyl group is
how does the heteroatom on carboxyls affect C=O bond strength?
lone pair donation weakens by conjugation
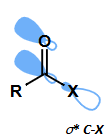
hyperconjugation of carbonyl lone pair with low lying C-X LUMO (σ*) strengthens bond
how does conjugation work at weakening bond? (draw resonance)
which atoms is this important for (O, Cl, N)?
most important for N as best donor
less important for O
not important for Cl - longer bond, weaker overlap
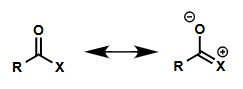
how does hyperconjugation strengthen C=O bond? (draw diagram)
which atoms is this important for (O, Cl, N)?
most important for Cl (lowest σ*)
less important for O
not important for N
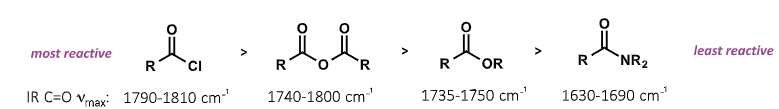
how does IR frequencies show reactivity of carboxyls?
higher frequency = more reactive
why are stronger C=O bonds more reactive?
conjugation weakens C=O bond, but stabilises entire functional group so harder to attack
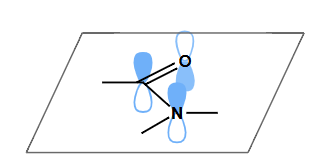
how does orbital overlap for amides work? what does this mean about their shape?
diagram?
N rehybridises from sp3 to sp2 to maximise orbital overlap
amides/peptides are planar
what is bond order for amides?
1.5 each for C-O and C-N
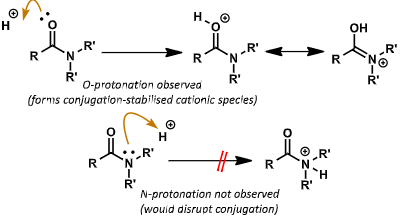
what does 1.5 bond order mean for amide nitrogen in terms of protonation?
N lone pair is not basic - tied up with conjugation
N protonation is not observed as this would disrupt the conjugation
O protonation is observed as LP is basic
what does 1.5 bond order mean for rotation in amides?
C-N rotation restricted
two rotational isomers (rotamers) seen
can be seen distinctly in H NMR spec
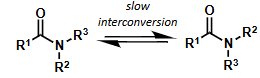
amide rotamers diagram
interconversion?
interconvert slowly at room temp
why can acids and esters be interconverted easily?
similar leaving groups
why can acid chlorides easily be converted to other groups while formation is difficult?
easy to displace better leaving group (Cl-)
why is it difficult to hydrolyse amides?
easier to displace better leaving groups
not good leaving group
how to convert acid chloride to c acid?
with water
why can’t esters be reduced with sodium borohydride?
what are they reduced with instead?
lone pair effect means esters are less reactive
lithium aluminium hydride
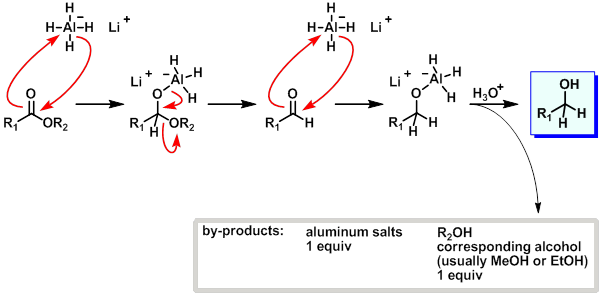
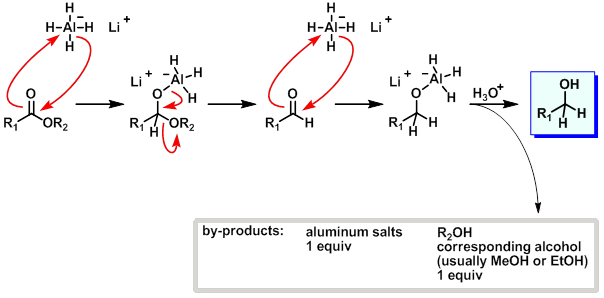
what are esters reduced to?
two alcohols
why do esters reduce to two alcohols?
tetrahedral intermediate is unstable and collapses to give an aldehyde
= more reactive to LiAlH4 so reacts further
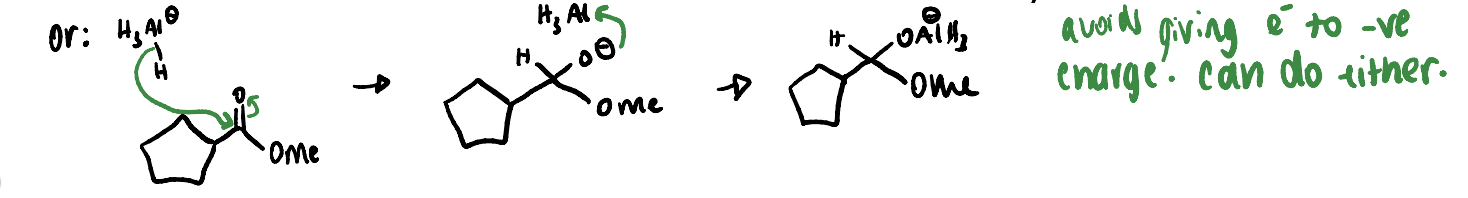
what is mechanism for ester reduction using AlH4?
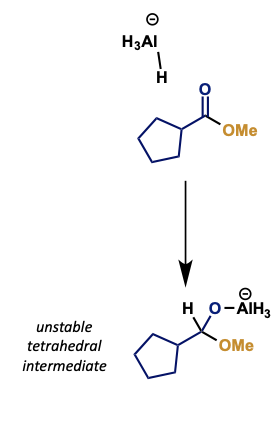
what is alternative mechanism for this step?
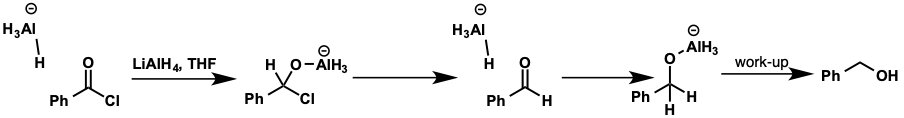
what does acid chloride reduce to?
what is intermediate?
reduces to alcohol
intermediate is aldehyde so reacts further with reducing agent
acid chloride reduction mechanism
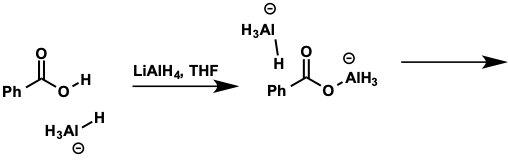
aluminium hydride anion is basic - what is first step of reduction of carboxylic acids?
deprotonation to give aluminium carboxylate
how is reduction of amines different to esters?
C-N remains intact
O is lost to give amine
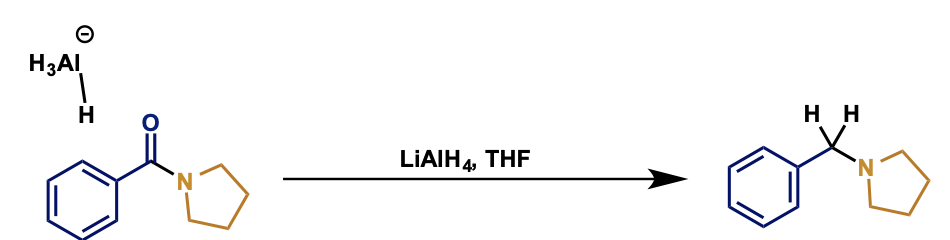
what is the mechanism?
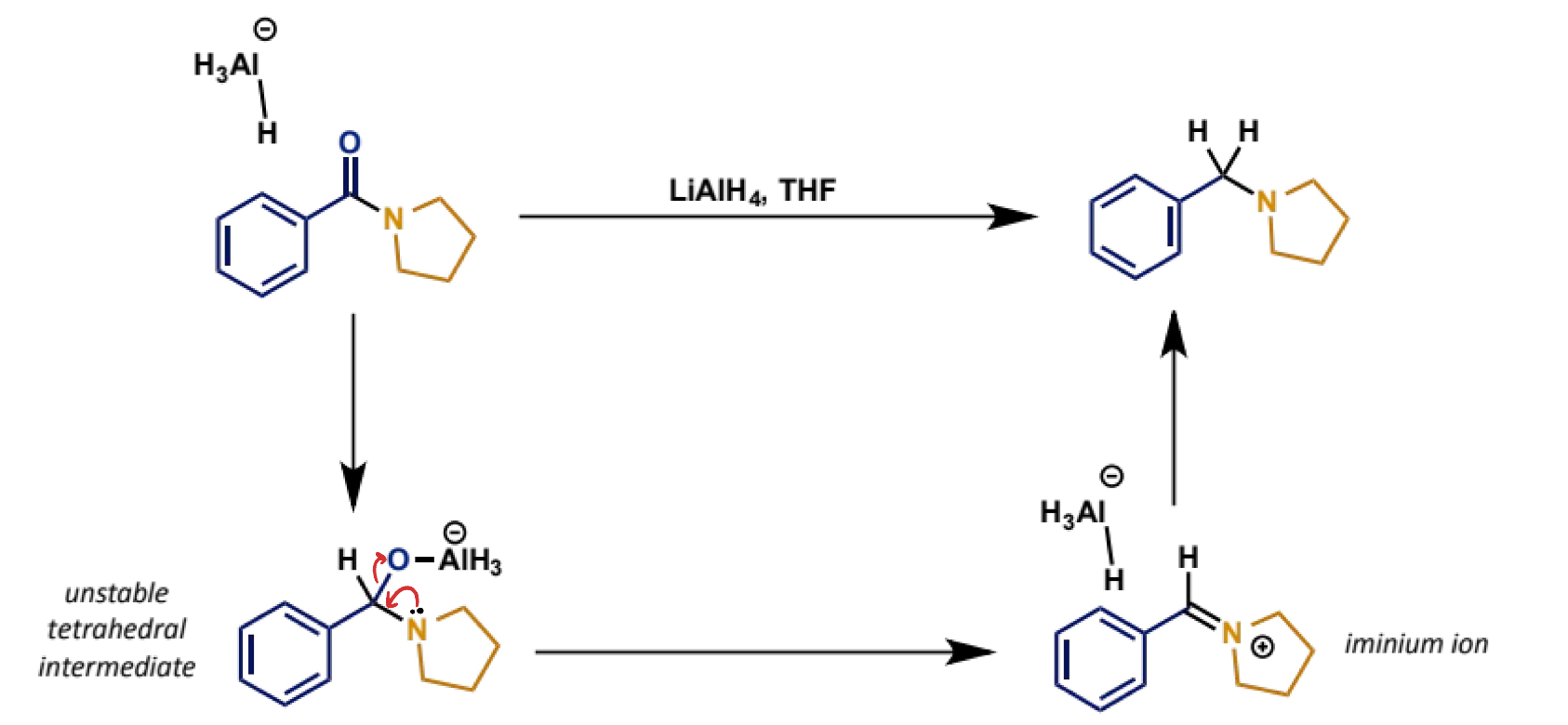
why is C-N bond not broken in reduction of amines?
alkoxide is better leaving group than nitrogen group
N atom more nucleophilic than O
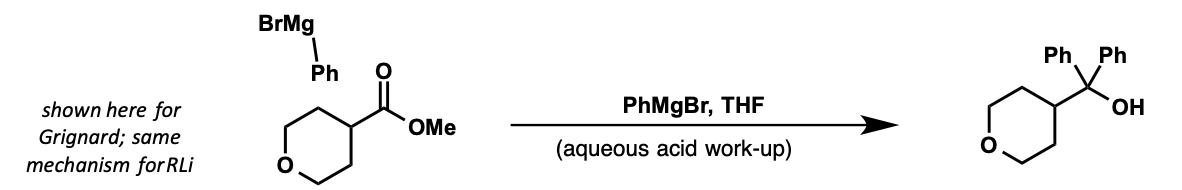
what does substitution by organometallics to ester lead to?
what is intermediate?
tertiary alcohol
ketone intermediate
what is the mechanism?
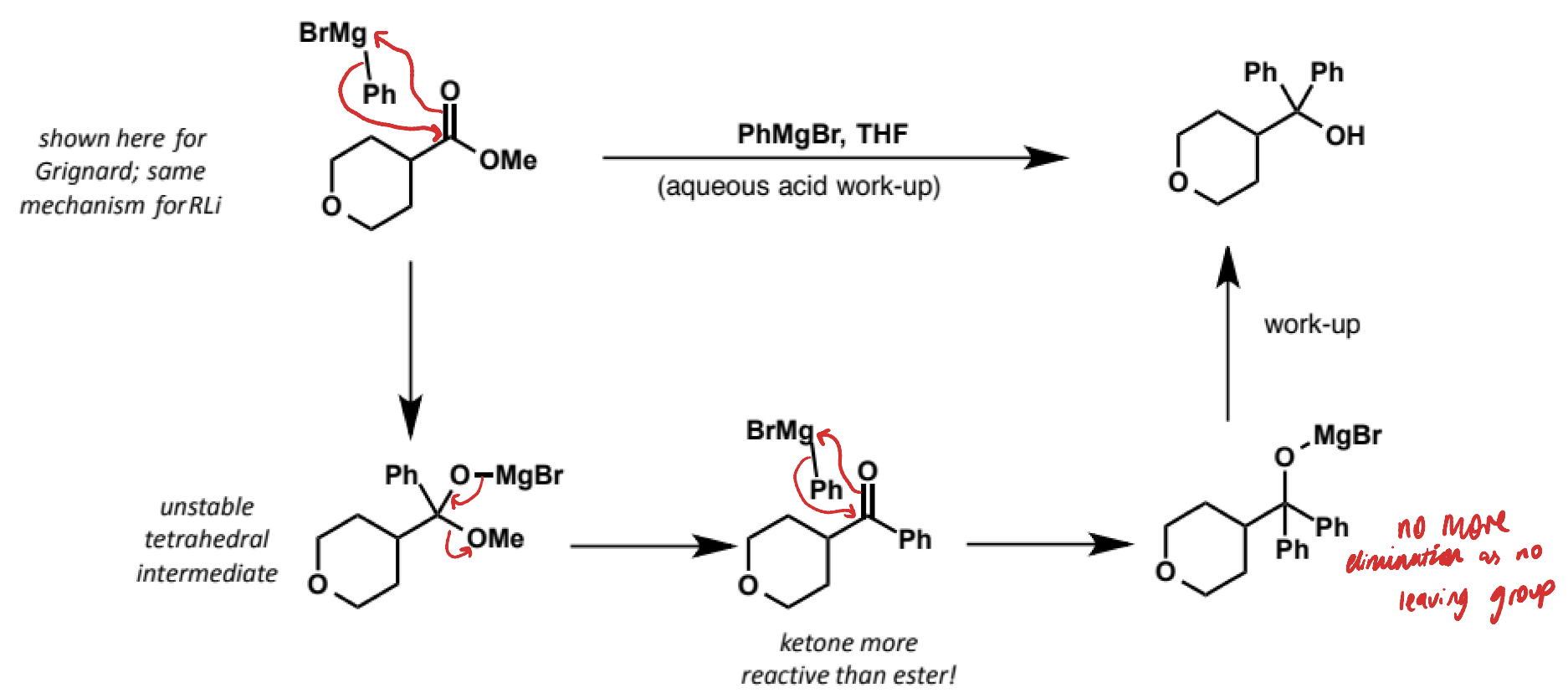
why does reaction of acid chloride and organometallics give mixtures of ketone and tertiary alcohol?
acid chlorides more reactive than esters
similar reactivity to ketones
amide reaction with organometallics
is there further addition?
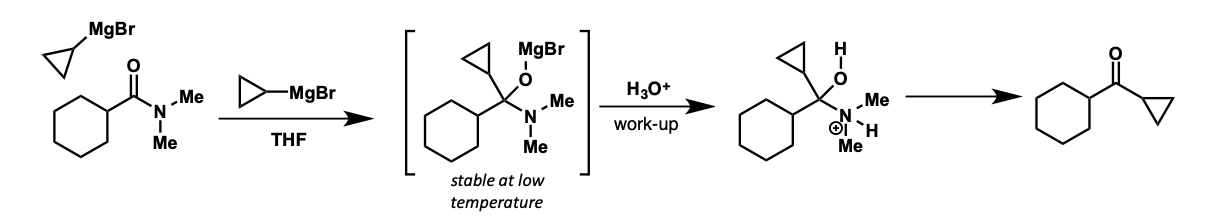
how can ketone be made?
tetrahedral intermediates reasonably stable at low temperatures
protects from further addition
quenched with water = ketone product
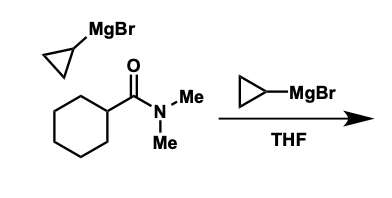
what is the intermediate formed?
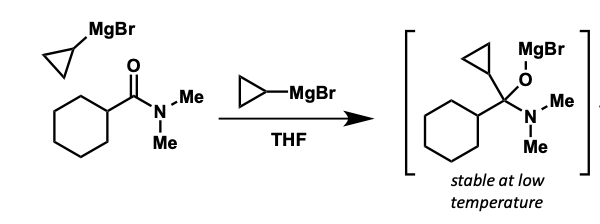
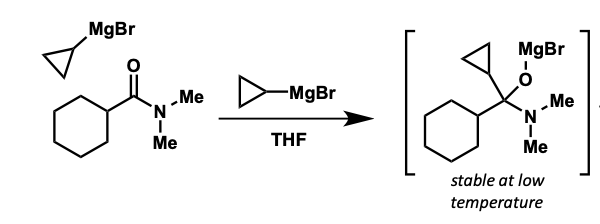
how is ketone product formed after this?
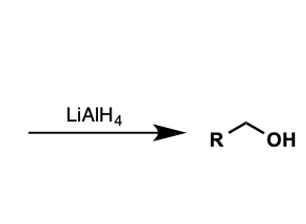
what molecules convert to primary alcohols with reduction by LiAlH4?
c acid
ester
acid chloride
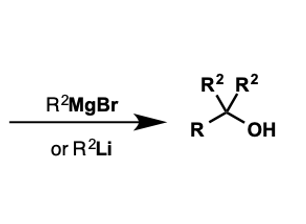
what molecules convert to tertiary alcohols by organometallics?
esters
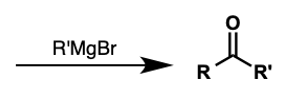
what molecules convert to ketones by Grignard?
amides
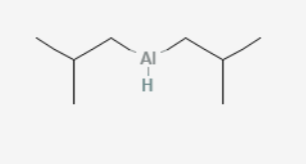
what molecules convert to aldehydes by DiBAL-H?
esters
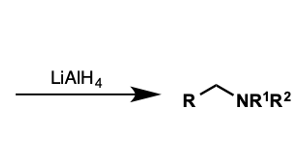
what molecules convert to amines by LiAlH4?
amides