Unit 19: Nuclear Chemistry - Radioactivity, Decay, Fission, and Applications
1/72
Earn XP
Description and Tags
Study Set For Unit 19, IB Chemistry SL. This does not include everything but covers much of the terms and process you need to know, but again does not include everything
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
What is nuclear chemistry?
The study of reactions involving changes in atomic nuclei.
Who began the study of nuclear chemistry?
French scientist Henri Becquerel in 1896.
What did Henri Becquerel discover?
That uranium rock is radioactive and can expose photographic plates even without sunlight.
Which elements did Marie Curie and Pierre Curie discover to be radioactive?
Radium and Polonium.
What does radioactivity refer to?
The spontaneous emission of particles or electromagnetic radiation by unstable nuclei.
How is the number of neutrons in an isotope calculated?
Number of neutrons = Atomic Mass - Atomic Number.
What is the isotopic symbol for gold with an atomic mass of 198?
Au-198 or 79/198Au.
What is the neutron to proton ratio for stable atoms of low atomic number?
Close to 1.
What are the 'magic numbers' in nuclear stability?
2, 8, 20, 50, 82, or 126 protons or neutrons.
What is the belt of stability?
A plot showing the most stable nuclei based on neutron vs. proton numbers.
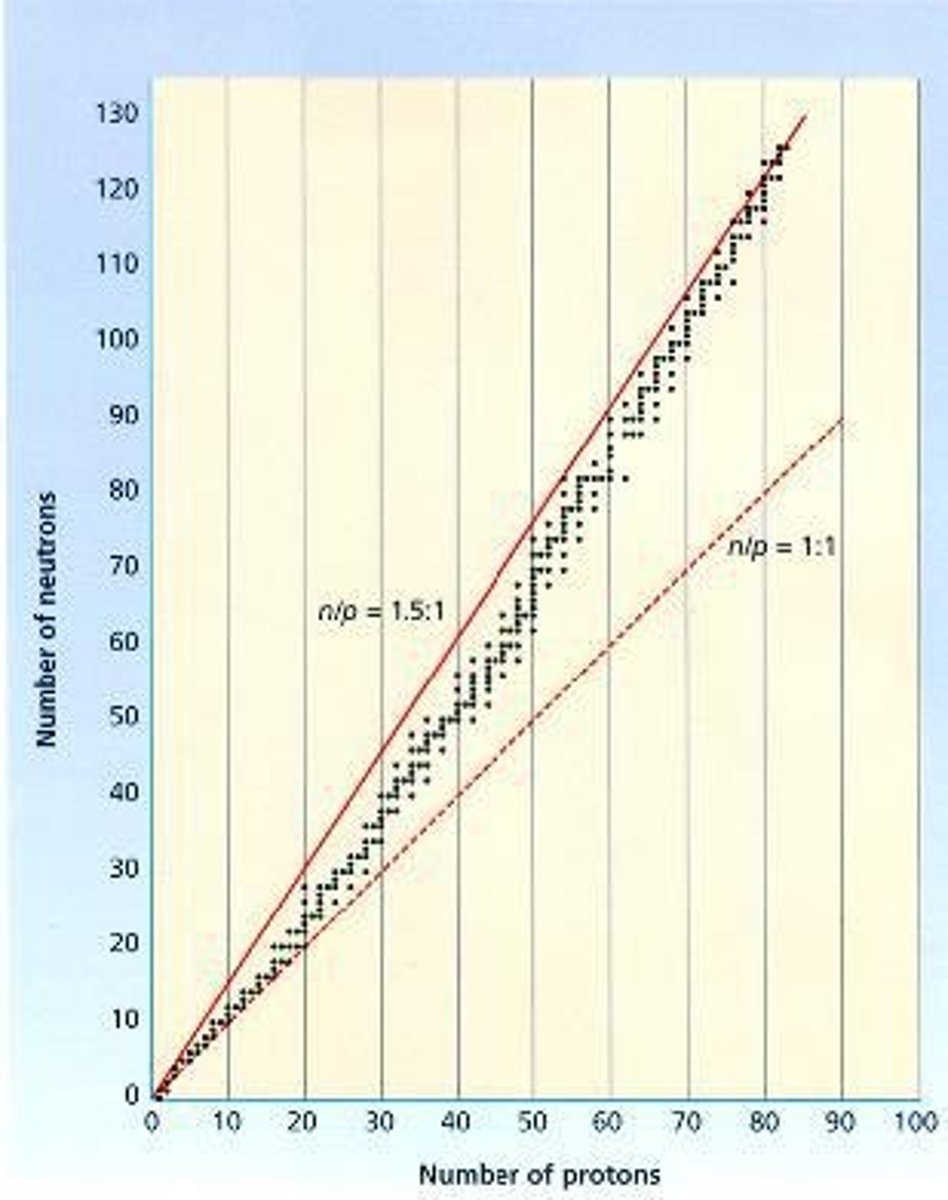
What are the three most common types of radioactive decay?
Alpha, beta, and gamma decay.
What can stop alpha particles?
A piece of paper.
What is beta decay?
The emission of an electron from a nucleus.
What is a positron?
A particle with the same mass as an electron but with a positive charge.
What is gamma radiation?
Electromagnetic radiation emitted from unstable nuclei.
What is electron capture?
The process where a nucleus captures an electron from its electron cloud.
What is nuclear binding energy?
The energy required to break up a nucleus into its component protons and neutrons.
What is mass defect?
The difference between the mass of an atom and the sum of the masses of its protons and neutrons.
What happens during alpha decay?
An unstable nucleus emits an alpha particle, reducing its atomic number by 2.
What is the significance of the law of conservation of mass in nuclear reactions?
It must be observed in all decay processes.
What is the result of beta decay?
A neutron is converted into a proton, emitting an electron.
What does positron emission typically occur in?
Nuclei below the belt of stability.
What is the role of a neutrino in electron capture?
It is emitted when an electron is captured by a proton in the nucleus.
What is a radioactive series?
A series of nuclear decay reactions that begins with an unstable isotope and ends with a stable isotope.
What is Einstein's famous equation that relates mass and energy?
E = mc², where E is energy, m is mass, and c is the speed of light.
What does ΔE represent in nuclear reactions?
ΔE represents the energy difference between products and reactants.
How is nuclear binding energy calculated?
Nuclear binding energy is calculated using ΔE = (Δm)c², where Δm is the mass defect.
How is nuclear binding energy per nucleon calculated?
It is calculated by dividing the total binding energy by the number of nucleons.
What is the significance of the curve of nuclear binding energy per nucleon vs. mass number?
It shows that mid-size nuclei are the most tightly bound and stable.
What are the two processes that can occur in nuclear reactions for stability?
Fission (splitting heavy nuclei) and fusion (combining small nuclei).
What does first order kinetics refer to in radioactive decay?
It describes the decay process where the rate is proportional to the number of atoms present.
What is the decay constant (λ) in radioactive decay?
It is related to the half-life (t½) by the equation λ = 0.693/t½.
What is the half-life of Tl-206?
4.20 minutes.
What is the first application of nuclear fission?
The development of the atomic bomb.
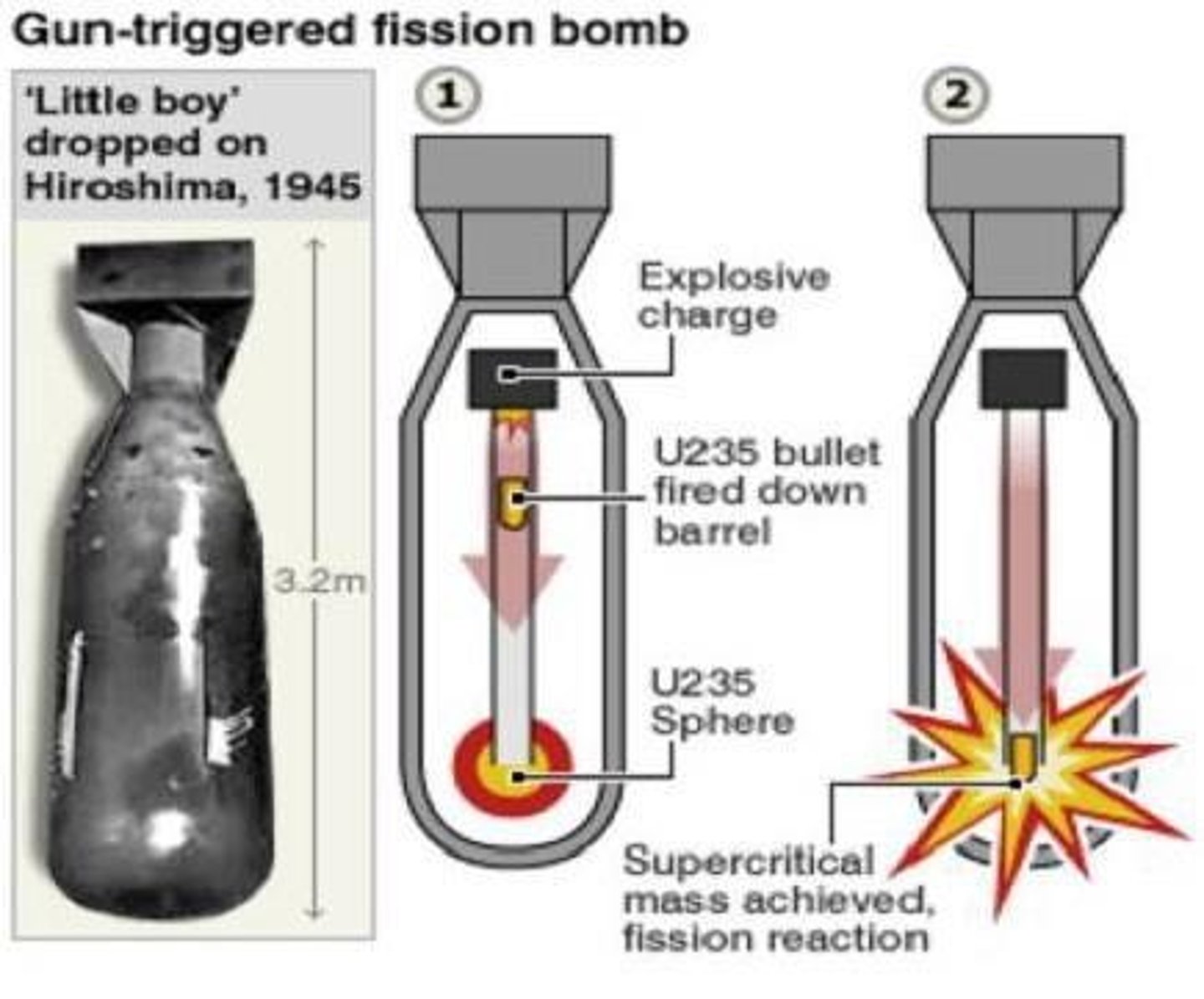
What is critical mass in nuclear fission?
The minimum mass needed to sustain a chain reaction.
Who first achieved nuclear fission and in what year?
Otto Hahn and Fritz Strassmann in late 1938.
What is the role of moderators in nuclear reactors?
Moderators slow down fast-moving neutrons produced during fission.
What are control rods used for in nuclear reactors?
Control rods absorb neutrons to regulate the fission process.
What is the difference between an atomic bomb and a nuclear reactor?
In a nuclear reactor, the chain reaction is controlled at all times.
What is the byproduct of nuclear fission that has a long half-life?
Strontium-90 (Sr-90).
What is the energy produced by a small atomic bomb equivalent to?
20,000 tons of TNT.
What is enriched uranium?
Uranium with a higher percentage of U-235, used in bombs and reactors.
What process is used to produce enriched uranium?
Gaseous diffusion, based on Graham's Law of Effusion.
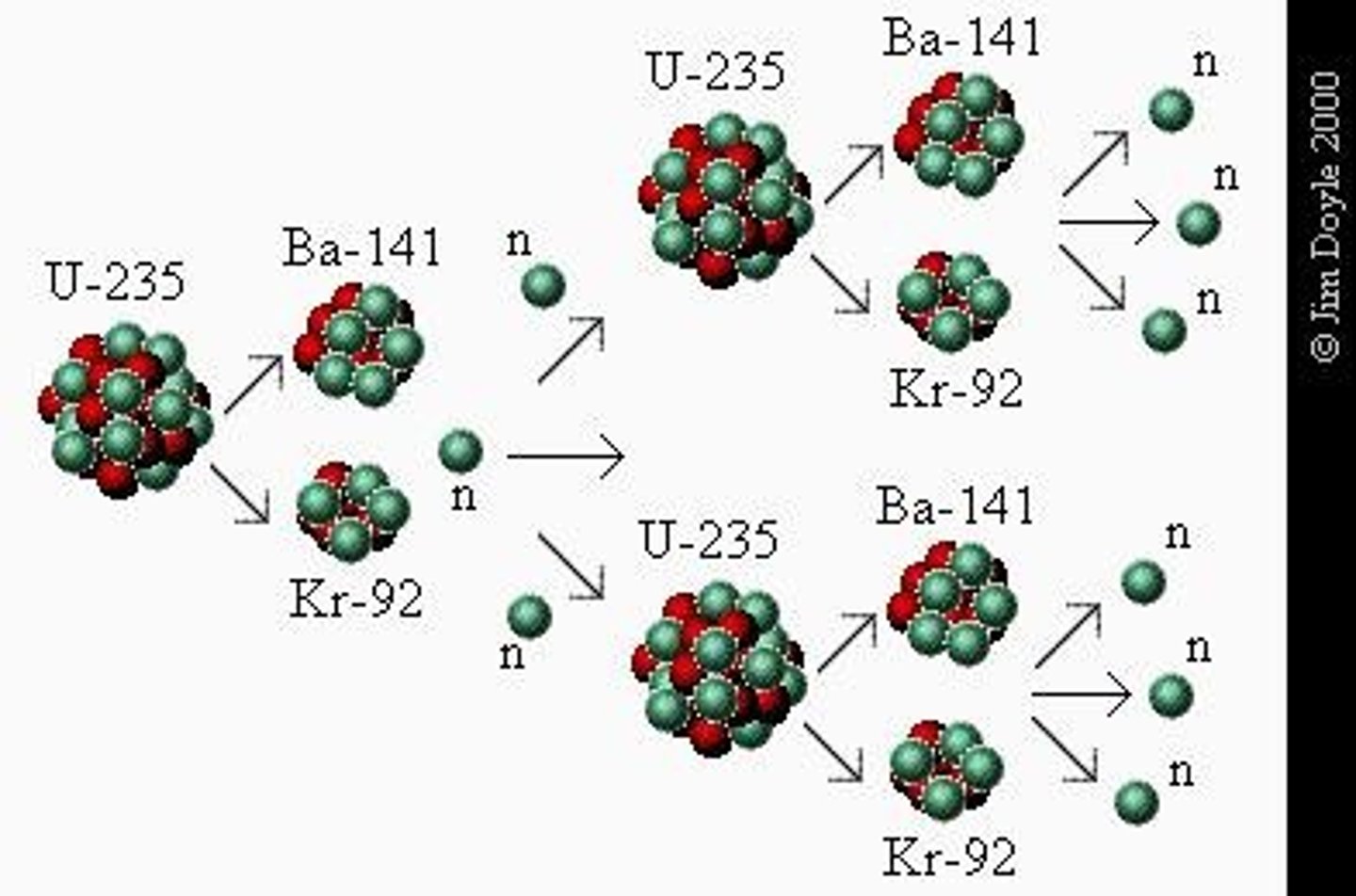
What is the significance of U-235 in nuclear reactions?
It produces more neutrons than are originally captured, enabling chain reactions.
What happens during the fission of U-235?
It splits into lighter nuclei and releases a large amount of energy.
Who contributed to the understanding of nuclear fission alongside Otto Hahn?
Lise Meitner and her nephew Otto Frisch.
What is the term for the self-sustaining sequence of nuclear fission reactions?
Chain reaction.
What is the relationship between nuclear binding energy and stability?
Higher binding energy per nucleon indicates greater stability of the nucleus.
What are nuclear transmutations?
Nuclear reactions induced by striking a nucleus with a neutron or other nucleus.
Who carried out the first nuclear transmutation and in what year?
Rutherford in 1919.
What is the equation for the first nuclear transmutation involving N-14?
14N + α-4 → 17O + 1p.
What is a particle accelerator?
A device that uses electric and magnetic fields to increase the kinetic energy of charged particles.
What was the first trans-uranium element synthesized?
Neptunium in 1940.
What is the world's largest particle accelerator?
The Large Hadron Collider, located between France and Switzerland.
What are some positive uses of radioisotopes?
Radioactive dating, medical tracers, and food irradiation.
How is Carbon-14 produced?
By cosmic rays hitting nitrogen in the atmosphere, producing neutrons that bombard nitrogen nuclei.
What is food irradiation used for?
To prevent foodborne illness, preserve food, control insects, delay sprouting, and sterilize food.
What are the three sources of radiation approved for food irradiation?
Gamma rays, X-rays, and electron beams.
What types of foods are approved for irradiation in the United States?
Beef, pork, crustaceans, fresh fruits and vegetables, poultry, seeds for sprouting, shell eggs, shellfish, and spices.
What is the effect of radiation on living tissue?
It removes electrons from atoms and molecules, forming ions and radicals that can damage cells.
What is the current standard dosage of radiation for nuclear workers?
5 rem/year.
What dosage of radiation is likely to result in death within 2 weeks?
500 rem.
What is the difference between radioactive decay and nuclear transmutation?
Radioactive decay is a spontaneous process, while nuclear transmutation is induced by external factors.
What is a chain reaction in nuclear fission?
A process where the products of one fission event cause further fission events.
Which isotopes are practical for nuclear fission?
Uranium-235 and Plutonium-239.
What is nuclear fission?
The splitting of a heavy nucleus into lighter nuclei, releasing energy.
What is the function of a moderator in a nuclear reactor?
To slow down neutrons to sustain the fission chain reaction.
What is the binding energy per nucleon?
The energy required to disassemble a nucleus into its constituent protons and neutrons.
What is the balanced equation for the alpha decay of Americium-241?
241Am → 237Np + 4He.
What is the balanced equation for the beta decay of Uranium-238?
238U → 238Np + β.
What happens to the n/p ratio in unstable isotopes?
It may be too high or too low, leading to different decay processes for stability.
What is the process of electron capture?
An unstable nucleus captures an electron, converting a proton into a neutron.
What is positron emission?
A decay process where a proton is converted into a neutron and a positron is emitted.