Biology Test 5
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
_ help carry out chemical reactions
protein
_ important part of biological membrane
lipid
_ contains hydrogen, oxygen, nitrogen phosphorus, carbon
nucleic acid
_ transports substances In/out of cell
protein
_ is made of amino acids
protein
_ sugar and starches
carbs
_ stores gene info
nucleicacid
_ are water proof covering
lipids (fat)
_ fats are single bonded and solids
saturated
_ fats are double bonded and liquid
unsaturated
_ fats compose of 2+ bonds and are liquid
polyunsaturated
_ are a organic compound
carbon
_ refers to the chem of _
organic, carbon
_ can form STRONG covalent bonds with many elements including itself
carbon
_ has 4 valence electrons
carbon
_ atoms can bond with carbon
carbon
_ substance composed of 2+ elements
compound
_+ _ properties will differ from individual elements
chemical, physical
_ is mixture is same(uniform) throughout
homogeneous
_ mixture is different throughout
heterogeneous
_ mixture with lt substances even distributed
solutions
suspension
mixture of water + undissolved mats (ex: water in sand(
_ is amount of soluted dissolved in a measure fixed amount of solution
concentration
_ is no more solute will dissolve
saturated solution
_ water is the solvent universal solvent
aqueous solution
chohp are elements in _
nucleic acids
sugar group, phosphate group, nitrogen-containing bases make up _
nucleic acid
ribonucleic acid is
rna
_ forms bone and muscle
protein
_ help fight disease
protein
_ regulate cell process and controls rate of smth
protein
_ measures the concentration neutral
ph scale
a molecule with an uneven distribution of charge
polar molecule
attraction between molecules of the same substance (CREATES SURFACE TENSION)
cohesion
attraction between molecules of different substances (MENISCUS)
adhesion
oxygen, carbon, hydrogen, nitrogen mainly make up the
human body
what percent of oxygen is in human body
65%
what percent of carbon is in human body
18%
what percent of hydrogen is in human body
10%
what percent of nitrogen is in human body
3%
elements in living things in very small amounts is
trace elements
nucleus charge is
positive
bonds form when one or more electron are transferred from one atom to another are
ionic bond
_ compounds forms hydrogen ion in solution
acid
macromolecule formed when monomers join together
polymer
bond formed when electrons are shared between atoms
covalent bond
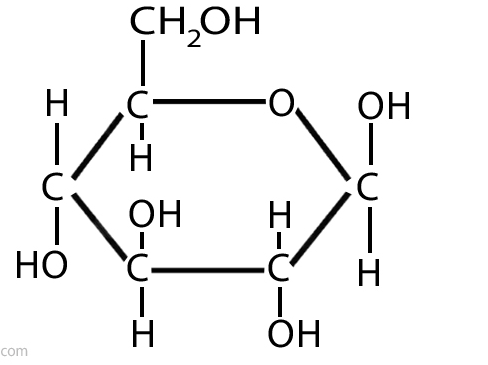
what is this
glucose
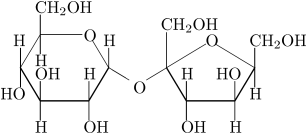
what is this
surcose
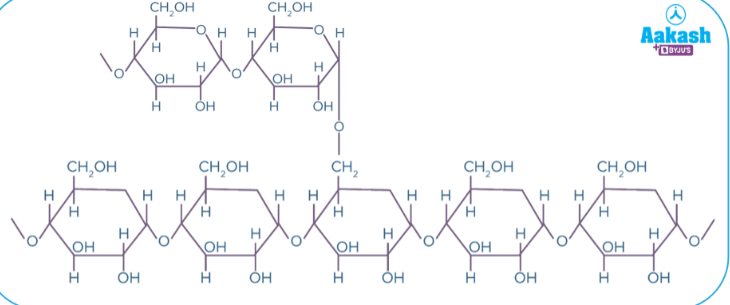
what is this
glycogen
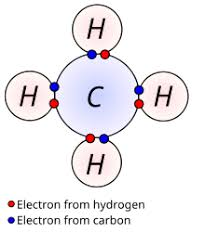
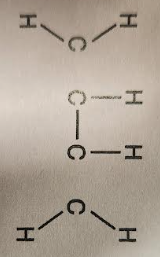
name the carbon compound
methane
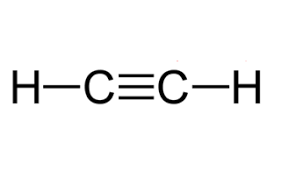
what carbon compound is this
acetylene
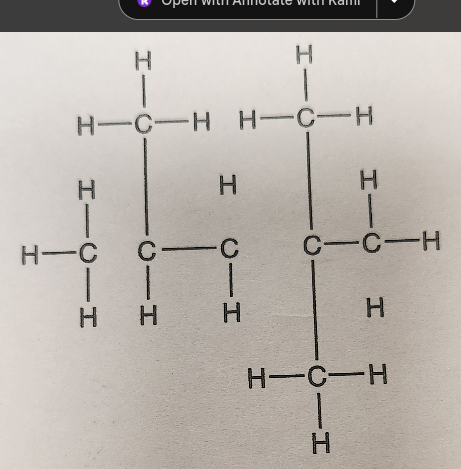
name the compound
isooctane
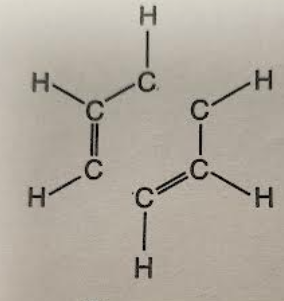
name the carbon compound
butadiene
name the carbon compound
benzene
relatively weak bond that hold large molecules together
hydrogen bond
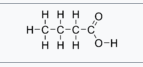
what is this
butyric acid
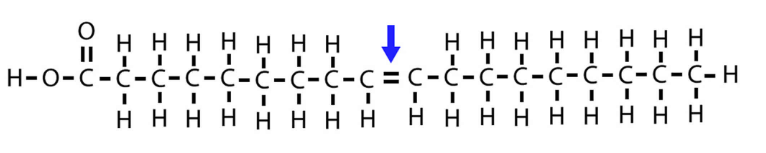
what is this
oleic acid
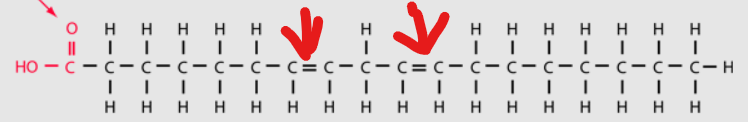
what is this
linoteic acid
what type of macromolecule is an enzyme
protein
0-6 on ph scale is
acidic
7 on ph scale is
neutral
8-14 on ph scale is
basic
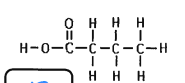
what is this
lipid
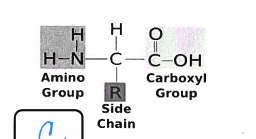
what is this
protein
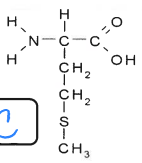
what is this
protein
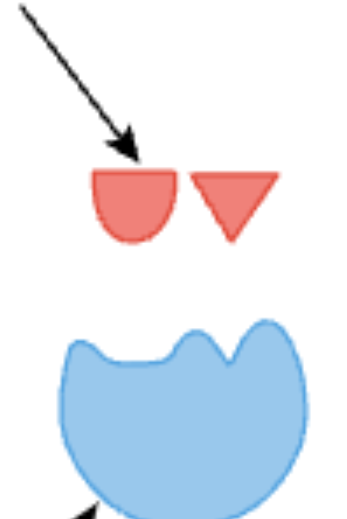
what is this
substrate
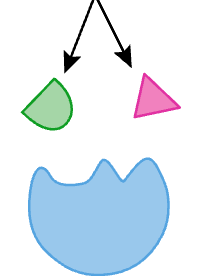
what is this
products
what is the end result of a chemical reaction
products