Tertiary structure of protein
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
43 Terms
What is the native state of a protein?
The native state is the folded 3D tertiary structure of a protein, which is required for its function.
What happens when a protein undergoes denaturation?
Denaturation unfolds the protein, causing it to become unstructured or aggregated, and often irreversible. The protein typically loses its function.
What are common ways to denature proteins?
Heat, disruptive solvents, and harsh detergents (like SDS) are common methods to denature proteins.
What does tertiary structure refer to in a protein?
Tertiary structure is the overall 3D pattern of folding of the entire polypeptide chain.
What is the simplest possible tertiary structure?
The simplest possible tertiary structure is continuous secondary structure.

What is the structure of α-keratin?
α-keratin is composed entirely of α-helices.
What is the structure of fibroin?
Fibroin (a type of β-keratin) is composed entirely of anti-parallel β-sheets.
What is unique about the structure of collagen?
Collagen has a unique triple-helix structure, found in tendons, bones, and connective tissue.
requires repeating units of three amino acids: Gly-Pro-X.
What type of structure do most proteins have?
Most proteins are globular, meaning their polypeptide chains fold back on themselves and reqiures breaks in the secondary structure.
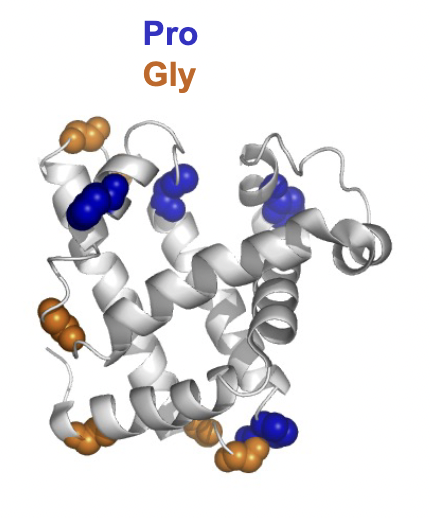
Why does globular protein folding require "breaks" in secondary structure?
Folding requires breaks because secondary structures like α-helices and β-sheets are rigid, and flexible loops or turns are needed to change direction for folding.
secondary structure breakers GPNDS are used for this allowing for flexible loops and turns to allow for folding.
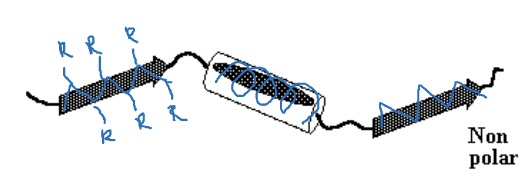
hydrophobic effect's role in protein folding
The hydrophobic effect drives protein folding by causing non-polar amino acids to be enclosed in the protein core, away from water.
polar main acids form the outer layer of the protein interacting through hydrogen bonds with water or ions in solution.
Which of the following amino acids is most likely to be
buried in the interior of a protein?
A. Aspartate
B. Threonine
C. Leucine
D. Lysine
C
How do amino acids pack together in a protein?
Amino acids pack together like a jigsaw puzzle, with their side chains interlocking to maximize close atom-to-atom contacts. This interlocking is caused by weak van der waal forces/London dispersion (0.1 to 1kj/mol per contact)
How do good and poor fits affect protein structure?
A good fit between amino acid side chains creates hundreds of close contacts, helping to hold the structure together, while a poor fit creates fewer contacts and weakens the structure.
Summary: How does a protein fold?
A protein folds based on its amino acid sequence. "Breaker" amino acids create flexible sections, while non-polar amino acids fold inward and polar ones stay on the outside. The pattern of side chains helps secondary structures like helices fit together, and a balance of forces leads to the stable, functional shape of the protein.
What are the main patterns of protein folded in tertiary structure?
Proteins generally fold into three main patterns:
Mostly a-helical segments
Mostly b-strand segments
Alternating a-helical and b-strand segments.
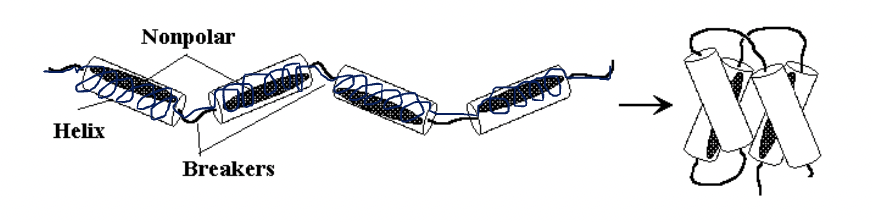
What happens when a protein consists mostly of a-helix-forming amino acids?
It folds into an a-helix bundle.
What is the role of non-polar amino acids in an a-helix bundle?
Non-polar amino acids placed every 3 or 4 residues form a non-polar patch or stripe that folds to the inside of the bundle.
e.g. PPNPPNNP
How are amino acids that prefer b-sheet arranged in an a-helix bundle?
They are scattered throughout the a-helix bundle, but still present.
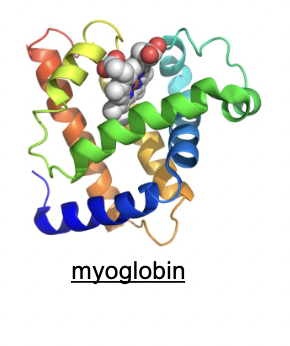
Can you give an example of a protein with an a-helix bundle structure?
Myoglobin is a well-known example, consisting of 8 a-helix sections.
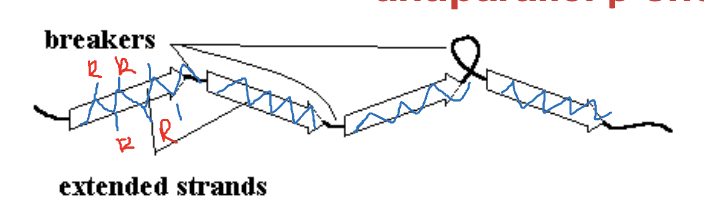
What happens when a protein has a majority of β-sheet-forming amino acids?
It folds into an antiparallel β-sheet.
Why is the antiparallel β-sheet more stable than the parallel β-sheet?
The hydrogen bonds are arranged in a straight line, making it more stable.
How do side chains project in a β-sheet?
Side chains project out of the sheet, with odd-numbered side chains on one side and even-numbered ones on the other.
Greek key motif
The Greek key motif is a pattern that consists of a continuous line that curves and bends in a way that creates a symmetrical and interlocking design, associated with β-sheet arrangements in proteins.
How can the sides of a β-sheet differ?
The sheet can be non-polar on one side and polar on the other side.
What happens when a β-sheet is polar on one side and non-polar on the other?
The β-sheet wraps around to enclose the non-polar face inside, forming a stable structure.
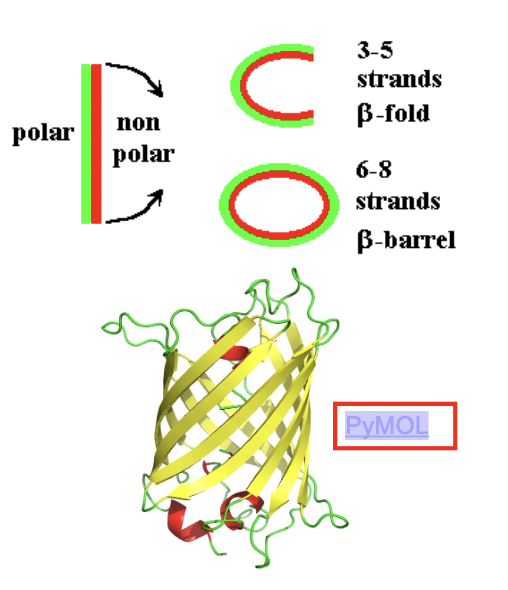
A small sheet (3-5 strands) makes an open fold, while a larger sheet (6-8 strands) wraps around to form an antiparallel β-barrel (hollow shaped barrel)
example of protein that has a B-sheet and is polar on one side and non polar on the other
green flueorescent protein
What sequence can form a parallel β-sheet?
Sequences that alternate between β-strand and α-helix (b-a-b-a) can form a parallel β-sheet.
How are the strands arranged in a parallel β-sheet?
The strands in a parallel β-sheet all run in the same direction, with helical sections connecting them.
parallel beta sheets are less stable because of their angled hydrogen bonds.
usually burried in the centre of proteins, mostly made up of non-polar AA
What happens if all the helices lie on one side of the sheet?
If all the helices lie on one side of the sheet, the sheet wraps around to form a parallel αβ-barrel.
where the central structure is a beta sheet forming a barrel surrounded by alpha helices
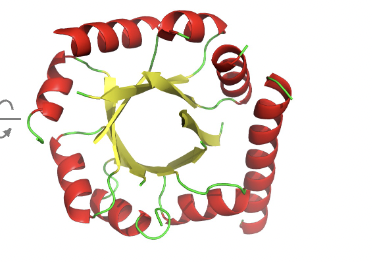
Can you give an example of a parallel αβ-barrel protein?
enzyme triose phosphate isomerase.
What is the structure when helices are on both sides of the sheet?
The structure is a parallel αβ-sandwich.
where we see the sandwich filling is the non-polar β-sheet between two layers of α-helix.
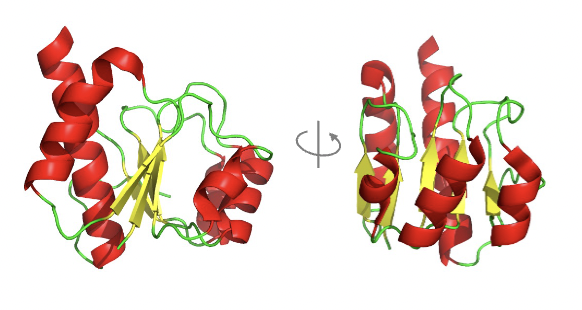
b sheet is often twisted for better packaging
Can you give an example of a protein with a parallel αβ-sandwich structure?
enzyme LDH
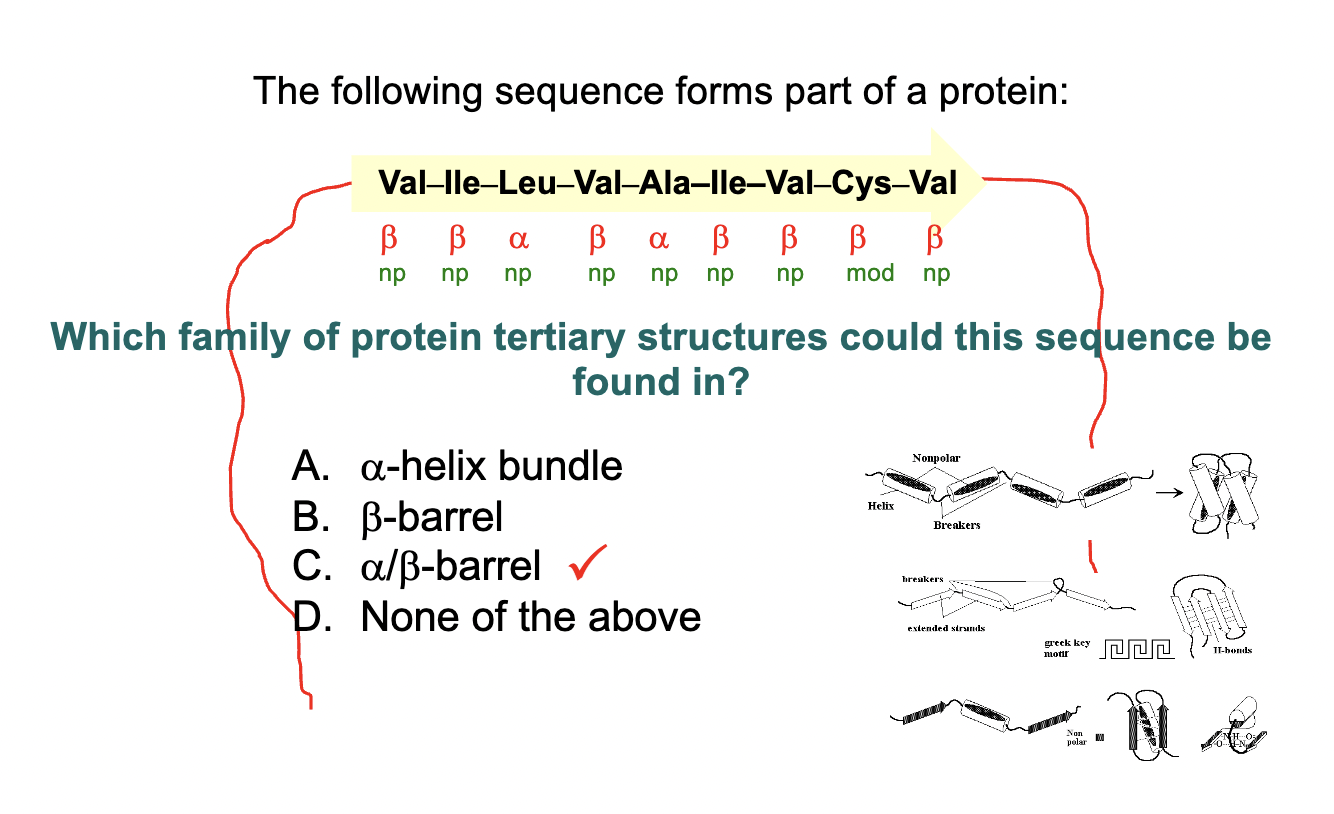
Explain this
Non-polar amino acids tend to cluster together in the interior of the protein, away from the aqueous environment
In an αβ-barrel, the α-helices typically surround the β-sheet structure, forming a barrel shape. The arrangement of helices surrounding the sheet helps stabilize the structure and shields the hydrophobic core.
hat are protein domains?
Protein domains are independent folding sections of a protein, often 10-20 kDa in size. They can adopt different folding patterns and contribute to the overall structure and function of larger proteins.
How do larger proteins typically fold?
Larger proteins fold into multiple domains, typically in sections of 10-20 kDa. For example, a 50 kDa protein might have 3 or 4 domains, each with its own folding pattern.
What does it mean that larger proteins are modular in nature?
Larger proteins are modular because they consist of several distinct domains, each of which may fold independently and contribute a specific function to the overall protein structure.
Give an example of a protein with multiple domains.
Lactate dehydrogenase is an example of a protein with two αβ-sandwich domains.
Protein stability is dictated by the following bonds
Covalent bonds (specifically peptide bonds) link amino acids in a chain and define the sequence of the protein.
Backbone hydrogen bonds stabilize the secondary structure (like α-helixes and β-sheets).
Non-covalent interactions dictate protein folding and stability:
The hydrophobic effect places non-polar amino acids in the protein's core, away from water, contributing to about 50% of the protein's stability.
Van der Waals interactions (London dispersion forces) are weak attractions between atoms in close contact and further stabilize the folded structure by maximizing atom-to-atom contact.
Hydrogen bonds (H-bonds) form between donors and acceptors that line up in the folded protein.
Ion pairs (salt bridges) are strong electrostatic interactions between negative side chains and positive side chains nearby in the folded protein.
Polar interactions help stabilize folding, but they make a smaller contribution since most polar groups face the protein's exterior, interacting with ions or water in the surroundings.
Which interaction contributed most to the formation of seondary structure in proteins?
Hydrogen bonding
How do disulfide bonds form in proteins?
Disulfide bonds form when pairs of Cys–SH groups react with O₂, releasing H₂O, creating a strong covalent bond that helps stabilize the protein's tertiary structure.
this helps maintain folded shape of protein and add extra stability
few proteins have disufide bonds, mostly proteins designed to function outside of cell have these.
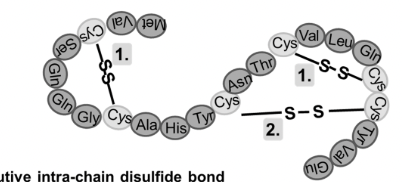
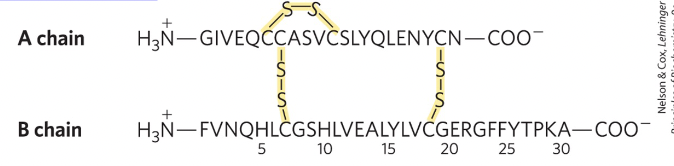
Primary structure of a protein contains all the information required to fom the protein’s secondary and tertiary structure true or false?
true
How was this proven that the primary structure contains all the info needed for the subsequent structures?
This was proven by Christian Anfinsen's experiment with ribonuclease. The enzyme was denatured in 8 M urea (which disrupted hydrophobic interactions) and 2-mercaptoethanol (which broke disulfide bonds). After removing these agents, the ribonuclease regained its native structure and biological activity, demonstrating that the amino acid sequence alone contains all the information required for the protein to refold into its functional form.